NPs Basic Information
|
Name |
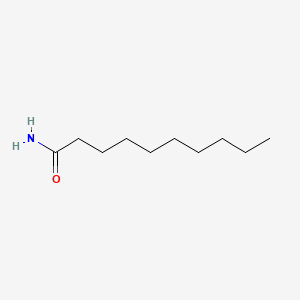
Decanamide
|
| Molecular Formula | C10H21NO | |
| IUPAC Name* |
decanamide
|
|
| SMILES |
CCCCCCCCCC(=O)N
|
|
| InChI |
InChI=1S/C10H21NO/c1-2-3-4-5-6-7-8-9-10(11)12/h2-9H2,1H3,(H2,11,12)
|
|
| InChIKey |
TUTWLYPCGCUWQI-UHFFFAOYSA-N
|
|
| Synonyms |
Decanamide; 2319-29-1; n-Decanamide; Decan-1-amide; Capramide; Decylamide; Decanoic acid amide; Decanamide-; N1468ZF07R; NSC-57564; 67700-97-4; Decanoylamide; UNII-N1468ZF07R; Capric acid amide; EINECS 219-029-7; ARMID 10; SCHEMBL43639; SCHEMBL19407773; CHEBI:38833; DTXSID80867310; DTXSID801022380; CAA31929; NSC57564; ZINC1688114; EINECS 266-921-7; LMFA08010005; MFCD00025541; NSC 57564; AKOS009159490; AS-56239; DB-046100; CS-0133974; FT-0624486; D89393; A878311; Q5801030
|
|
| CAS | 2319-29-1 | |
| PubChem CID | 75347 | |
| ChEMBL ID | NA |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 171.28 | ALogp: | 3.4 |
| HBD: | 1 | HBA: | 1 |
| Rotatable Bonds: | 8 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 43.1 | Aromatic Rings: | 0 |
| Heavy Atoms: | 12 | QED Weighted: | 0.559 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.602 | MDCK Permeability: | 0.00002920 |
| Pgp-inhibitor: | 0.01 | Pgp-substrate: | 0.008 |
| Human Intestinal Absorption (HIA): | 0.002 | 20% Bioavailability (F20%): | 0.851 |
| 30% Bioavailability (F30%): | 0.946 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.998 | Plasma Protein Binding (PPB): | 91.55% |
| Volume Distribution (VD): | 0.678 | Fu: | 12.92% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.873 | CYP1A2-substrate: | 0.644 |
| CYP2C19-inhibitor: | 0.247 | CYP2C19-substrate: | 0.09 |
| CYP2C9-inhibitor: | 0.244 | CYP2C9-substrate: | 0.705 |
| CYP2D6-inhibitor: | 0.016 | CYP2D6-substrate: | 0.136 |
| CYP3A4-inhibitor: | 0.044 | CYP3A4-substrate: | 0.081 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 7.374 | Half-life (T1/2): | 0.239 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.108 | Human Hepatotoxicity (H-HT): | 0.032 |
| Drug-inuced Liver Injury (DILI): | 0.042 | AMES Toxicity: | 0.014 |
| Rat Oral Acute Toxicity: | 0.032 | Maximum Recommended Daily Dose: | 0.015 |
| Skin Sensitization: | 0.633 | Carcinogencity: | 0.099 |
| Eye Corrosion: | 0.113 | Eye Irritation: | 0.936 |
| Respiratory Toxicity: | 0.038 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000472 | 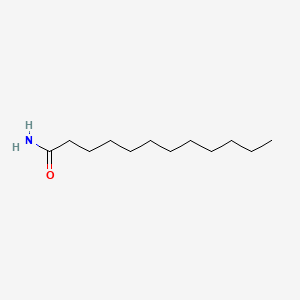 |
0.846 | D0Z5BC |  |
0.500 | ||
| ENC000687 | 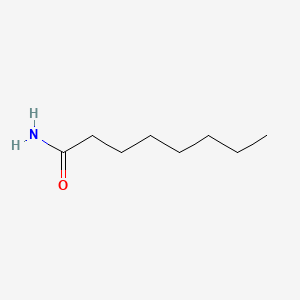 |
0.818 | D03ZJE | 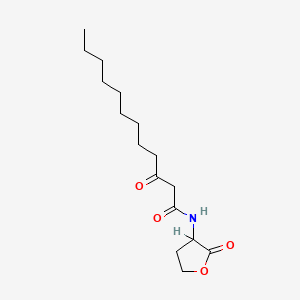 |
0.438 | ||
| ENC000265 | 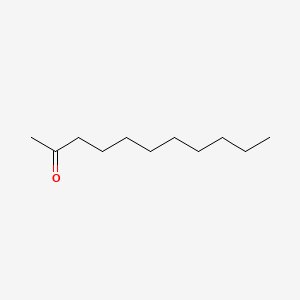 |
0.737 | D07ILQ |  |
0.431 | ||
| ENC000088 | 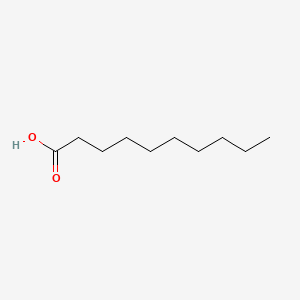 |
0.737 | D05ATI |  |
0.429 | ||
| ENC000270 | 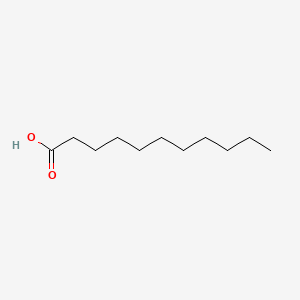 |
0.683 | D0E4WR | 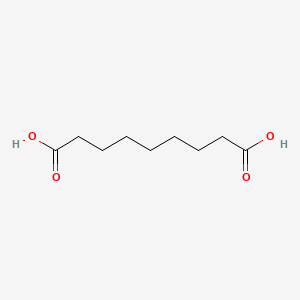 |
0.417 | ||
| ENC000249 |  |
0.683 | D0XN8C |  |
0.415 | ||
| ENC000487 |  |
0.683 | D0AY9Q |  |
0.415 | ||
| ENC000556 |  |
0.683 | D0O1PH |  |
0.414 | ||
| ENC000263 | 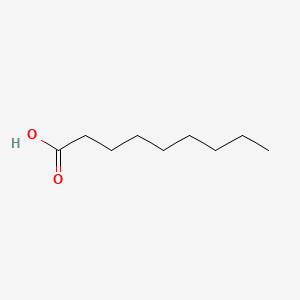 |
0.658 | D0Z5SM |  |
0.381 | ||
| ENC000451 | 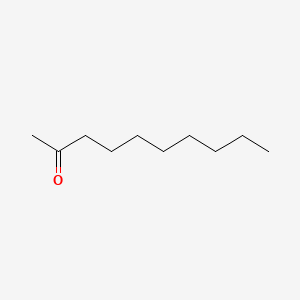 |
0.658 | D0O1TC | 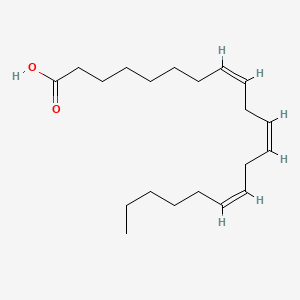 |
0.371 | ||