NPs Basic Information
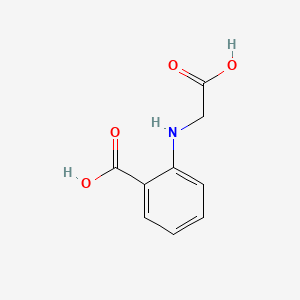
|
Name |
N-(Carboxymethyl)anthranilic acid
|
| Molecular Formula | C9H9NO4 | |
| IUPAC Name* |
2-(carboxymethylamino)benzoic acid
|
|
| SMILES |
C1=CC=C(C(=C1)C(=O)O)NCC(=O)O
|
|
| InChI |
InChI=1S/C9H9NO4/c11-8(12)5-10-7-4-2-1-3-6(7)9(13)14/h1-4,10H,5H2,(H,11,12)(H,13,14)
|
|
| InChIKey |
PJUXPMVQAZLJEX-UHFFFAOYSA-N
|
|
| Synonyms |
612-42-0; N-(2-Carboxyphenyl)glycine; N-(Carboxymethyl)anthranilic acid; 2-[(carboxymethyl)amino]benzoic acid; 2-((Carboxymethyl)amino)benzoic acid; 2-(carboxymethylamino)benzoic acid; Benzoic acid, 2-[(carboxymethyl)amino]-; Phenylglycine-o-carboxylic acid; 2-(Carboxymethyl-amino)-benzoic acid; Glycine, N-(o-carboxyphenyl)-; Anthranilic acid, N-(carboxymethyl)-; MFCD00020445; Benzoic acid, 2-((carboxymethyl)amino)-; 141865-09-0; 2-[(Carboxymethyl)Amino]-Benzoicaci; N-Phenylglycine-o-carboxylic acid; EINECS 210-311-5; NSC 80600; o-carboxyphenylglycine; Anthranilinoacetic acid; Cambridge id 5108438; Oprea1_043997; Oprea1_087556; CBDivE_014055; MLS000680124; BIDD:GT0666; SCHEMBL341645; CHEMBL443231; IFLab1_000341; DTXSID9060609; ZINC50907; HMS1412P11; HMS2745I07; NSC80600; BBL023541; NSC-80600; STK698970; AKOS000274214; CS-W010629; HY-W009913; IDI1_008560; NCGC00246622-01; AC-17985; BP-12709; SMR000324392; DB-080992; FT-0629270; (Carbomethoxymethyl)triphenylphosphoniumbromide; D78226; AH-034/04643009; Q27454068; 3RG
|
|
| CAS | 612-42-0 | |
| PubChem CID | 69161 | |
| ChEMBL ID | CHEMBL443231 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 195.17 | ALogp: | 1.7 |
| HBD: | 3 | HBA: | 5 |
| Rotatable Bonds: | 4 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 86.6 | Aromatic Rings: | 1 |
| Heavy Atoms: | 14 | QED Weighted: | 0.674 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -5.98 | MDCK Permeability: | 0.00001000 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.004 |
| Human Intestinal Absorption (HIA): | 0.086 | 20% Bioavailability (F20%): | 0.004 |
| 30% Bioavailability (F30%): | 0.626 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.22 | Plasma Protein Binding (PPB): | 61.49% |
| Volume Distribution (VD): | 0.197 | Fu: | 31.37% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.033 | CYP1A2-substrate: | 0.037 |
| CYP2C19-inhibitor: | 0.079 | CYP2C19-substrate: | 0.043 |
| CYP2C9-inhibitor: | 0.163 | CYP2C9-substrate: | 0.257 |
| CYP2D6-inhibitor: | 0.029 | CYP2D6-substrate: | 0.121 |
| CYP3A4-inhibitor: | 0.027 | CYP3A4-substrate: | 0.015 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 2.127 | Half-life (T1/2): | 0.857 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.057 | Human Hepatotoxicity (H-HT): | 0.281 |
| Drug-inuced Liver Injury (DILI): | 0.706 | AMES Toxicity: | 0.017 |
| Rat Oral Acute Toxicity: | 0.02 | Maximum Recommended Daily Dose: | 0.005 |
| Skin Sensitization: | 0.491 | Carcinogencity: | 0.016 |
| Eye Corrosion: | 0.027 | Eye Irritation: | 0.946 |
| Respiratory Toxicity: | 0.619 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000055 |  |
0.591 | D07HBX |  |
0.512 | ||
| ENC003916 |  |
0.509 | D0GY5Z |  |
0.490 | ||
| ENC000073 | 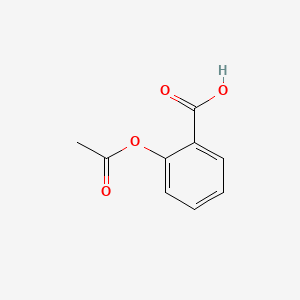 |
0.490 | D0N3UL | 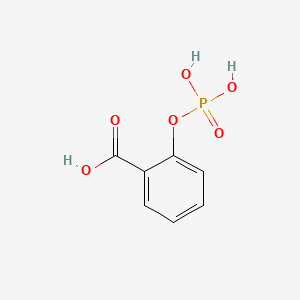 |
0.442 | ||
| ENC005325 | 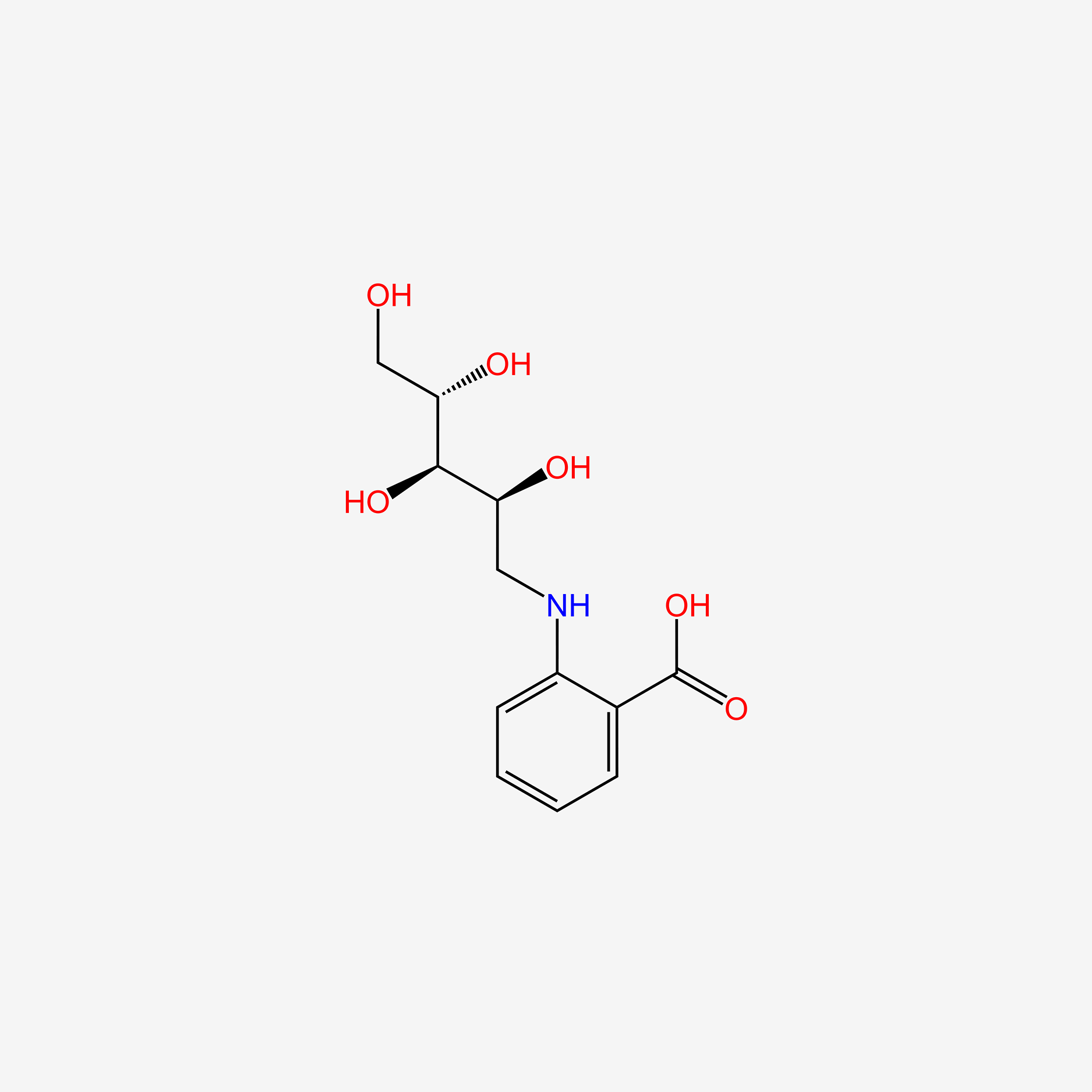 |
0.483 | D0G2MH | 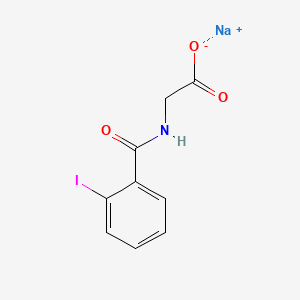 |
0.426 | ||
| ENC000301 | 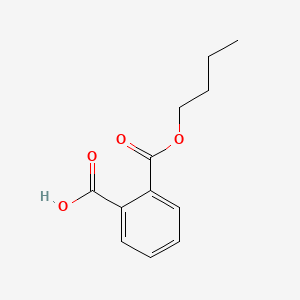 |
0.439 | D05FTJ | 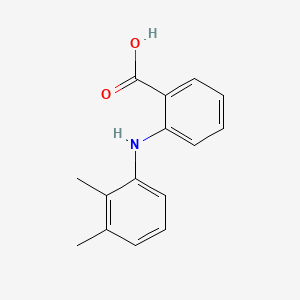 |
0.397 | ||
| ENC001443 | 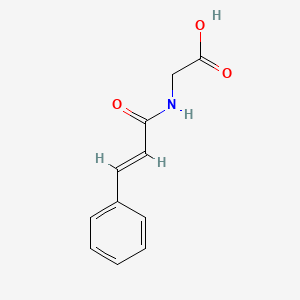 |
0.429 | D08IFL | 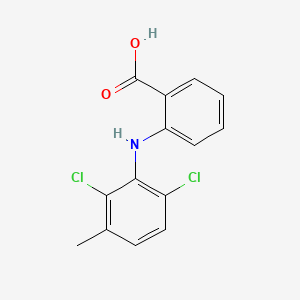 |
0.385 | ||
| ENC000409 | 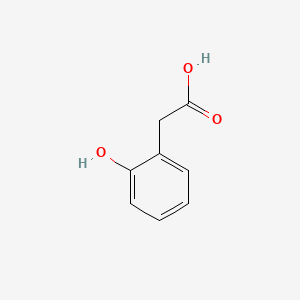 |
0.417 | D0F5ZM | 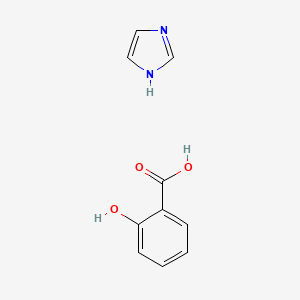 |
0.379 | ||
| ENC001333 |  |
0.388 | D0Y0JH | 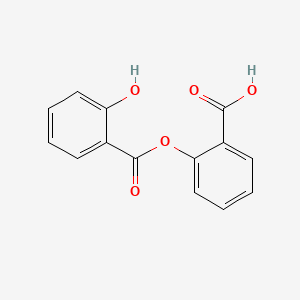 |
0.379 | ||
| ENC005757 | 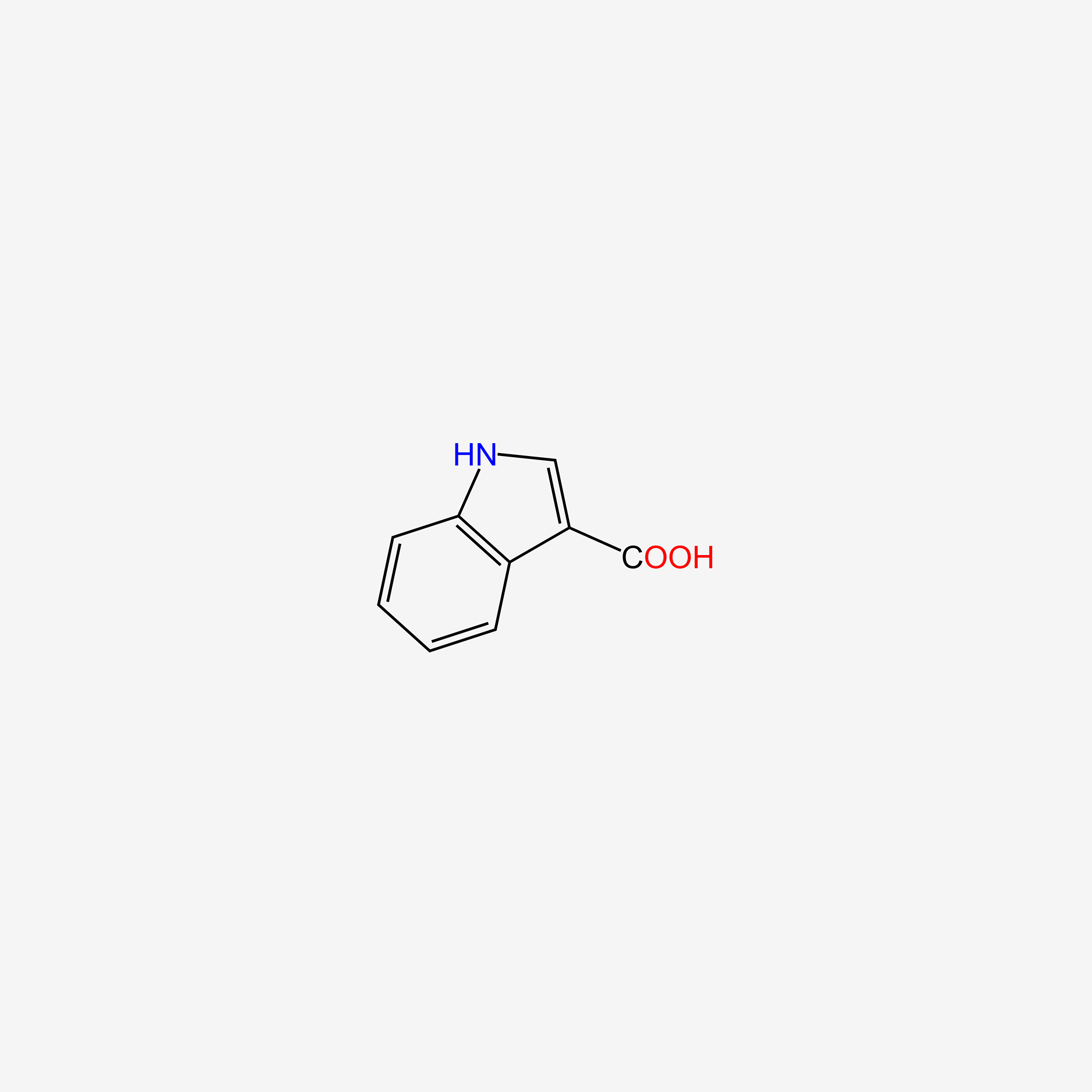 |
0.385 | D0B2WJ | 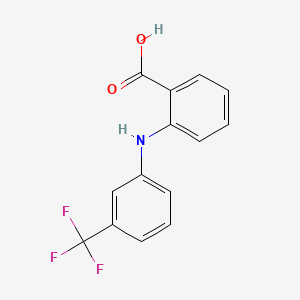 |
0.368 | ||
| ENC000108 | 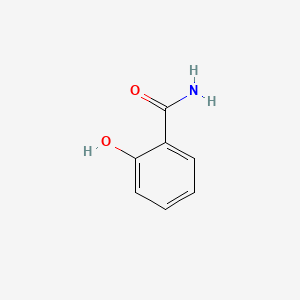 |
0.383 | D0E6OC | 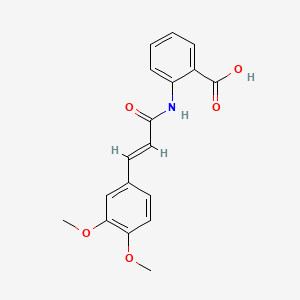 |
0.364 | ||