NPs Basic Information
|
Name |
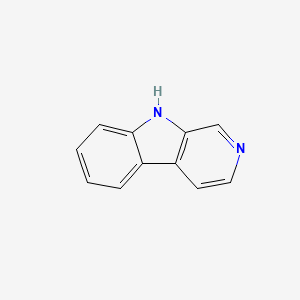
9H-Pyrido[3,4-B]indole
|
| Molecular Formula | C11H8N2 | |
| IUPAC Name* |
9H-pyrido[3,4-b]indole
|
|
| SMILES |
C1=CC=C2C(=C1)C3=C(N2)C=NC=C3
|
|
| InChI |
InChI=1S/C11H8N2/c1-2-4-10-8(3-1)9-5-6-12-7-11(9)13-10/h1-7,13H
|
|
| InChIKey |
AIFRHYZBTHREPW-UHFFFAOYSA-N
|
|
| Synonyms |
9H-Pyrido[3,4-B]indole; Norharman; Norharmane; 244-63-3; beta-Carboline; 2,9-Diazafluorene; Carbazoline; 9H-Beta-carboline; 2-Azacarbazole; 9H-Pyrido(3,4-B)indole; .beta.-Carboline; 2H-Pyrido[3,4-b]indole; CHEBI:109895; 244-63-3 (Free base).; MFCD00004956; MLS000069651; 94HMA1I78O; CHEMBL275224; 244-66-6; NSC-84417; SMR000058207; b-carboline; Carbazoline (VAN); Nor Harmane; CCRIS 6915; SR-01000000213; EINECS 205-959-0; NSC 84417; BRN 0128414; UNII-94HMA1I78O; Prestwick_363; Norharmane, 98%; Norharman, free base; 9H-Beta-carboline #; Norharman - free base; Norharmane, crystalline; Kinome_3628; Spectrum_001132; 9H-I(2)-Carboline; beta-carboline norharman; Opera_ID_1385; Spectrum2_000588; Spectrum3_000741; Spectrum4_001915; Spectrum5_000630; DSSTox_CID_1070; Epitope ID:140123; DSSTox_RID_75928; NCIOpen2_001217; DSSTox_GSID_21070; SCHEMBL25834; BSPBio_002322; KBioGR_002537; KBioSS_001612; 5-23-08-00220 (Beilstein Handbook Reference); cid_64961; MLS001148623; SPBio_000436; GTPL8222; DTXSID2021070; KBio2_001612; KBio2_004180; KBio2_006748; KBio3_001542; WLN: T B656 EN HMJ; ZINC66039; 2-Hydro-9-dehydro-beta-carboline; norharman hydrochloride monohydrate; HMS2233G10; HMS3369E13; ACT06124; BCP20998; NSC84417; (c)micro-Carboline; 2-Azacarbazole; Tox21_200083; BDBM50013811; CCG-38511; STL562246; AKOS015969732; CS-W008566; HY-W008566; SDCCGMLS-0003278.P003; SMP2_000349; NCGC00018245-01; NCGC00018245-02; NCGC00018245-03; NCGC00018245-04; NCGC00018245-05; NCGC00018245-06; NCGC00021302-03; NCGC00021302-04; NCGC00257637-01; CAS-244-63-3; DS-10932; SY050760; XN175452; DB-046456; FT-0610769; P1121; VU0239493-6; C20157; N-8700; N-8720; A817317; Q414226; SR-01000000213-3; SR-01000000213-4; BRD-K47467075-001-02-7; BRD-K47467075-001-03-5; BRD-K47467075-001-13-4; NRH
|
|
| CAS | 244-63-3 | |
| PubChem CID | 64961 | |
| ChEMBL ID | CHEMBL275224 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 168.19 | ALogp: | 3.2 |
| HBD: | 1 | HBA: | 1 |
| Rotatable Bonds: | 0 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 28.7 | Aromatic Rings: | 3 |
| Heavy Atoms: | 13 | QED Weighted: | 0.546 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.585 | MDCK Permeability: | 0.00002700 |
| Pgp-inhibitor: | 0.006 | Pgp-substrate: | 0.008 |
| Human Intestinal Absorption (HIA): | 0.008 | 20% Bioavailability (F20%): | 0.925 |
| 30% Bioavailability (F30%): | 0.399 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.966 | Plasma Protein Binding (PPB): | 87.49% |
| Volume Distribution (VD): | 1.976 | Fu: | 11.04% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.995 | CYP1A2-substrate: | 0.662 |
| CYP2C19-inhibitor: | 0.795 | CYP2C19-substrate: | 0.323 |
| CYP2C9-inhibitor: | 0.268 | CYP2C9-substrate: | 0.911 |
| CYP2D6-inhibitor: | 0.875 | CYP2D6-substrate: | 0.888 |
| CYP3A4-inhibitor: | 0.906 | CYP3A4-substrate: | 0.338 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 8.574 | Half-life (T1/2): | 0.666 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.051 | Human Hepatotoxicity (H-HT): | 0.49 |
| Drug-inuced Liver Injury (DILI): | 0.934 | AMES Toxicity: | 0.497 |
| Rat Oral Acute Toxicity: | 0.963 | Maximum Recommended Daily Dose: | 0.863 |
| Skin Sensitization: | 0.908 | Carcinogencity: | 0.116 |
| Eye Corrosion: | 0.105 | Eye Irritation: | 0.991 |
| Respiratory Toxicity: | 0.99 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC005053 | 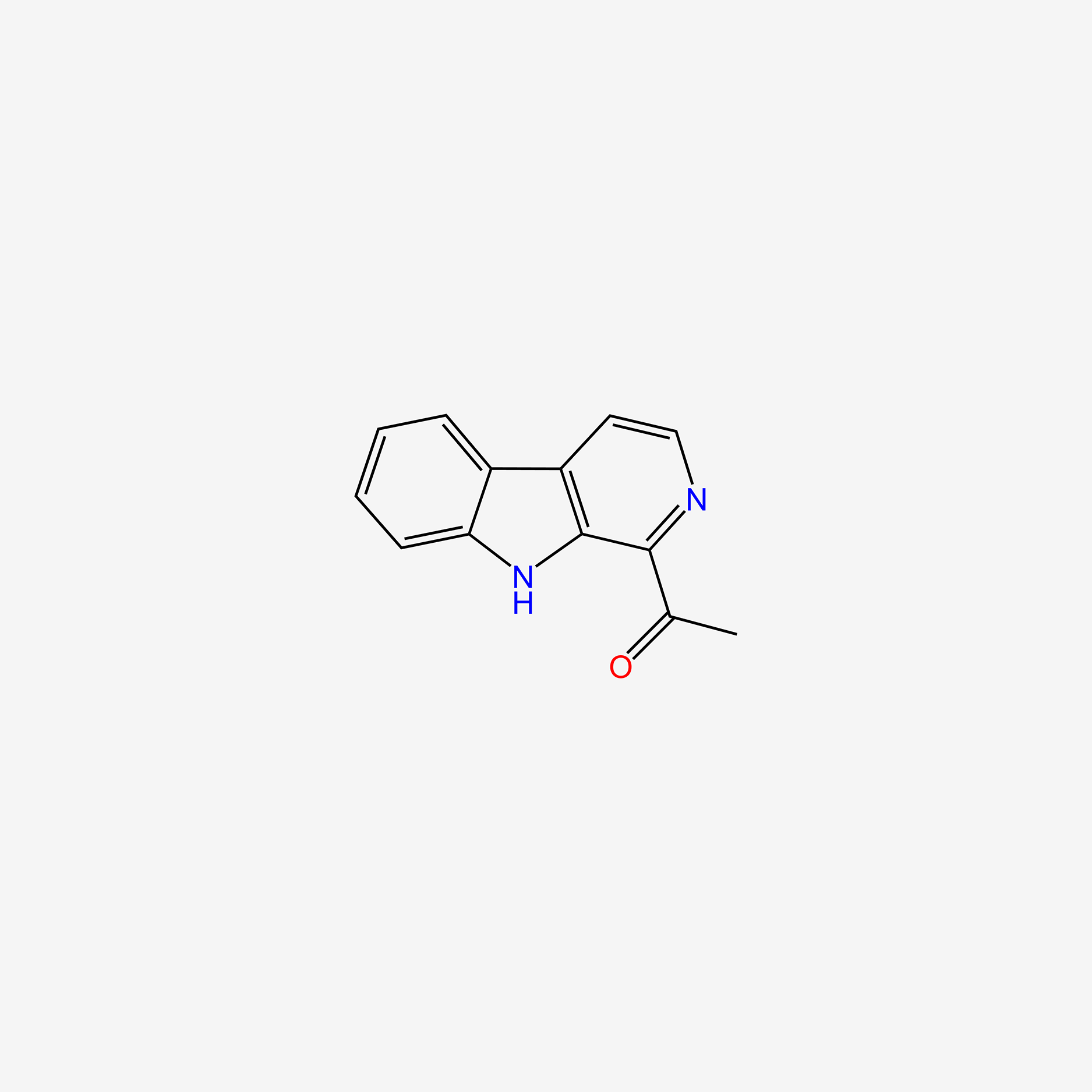 |
0.518 | D08QCJ | 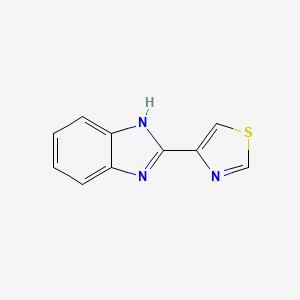 |
0.350 | ||
| ENC002323 | 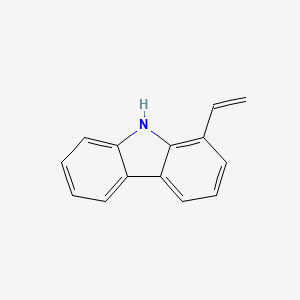 |
0.482 | D0O6IZ | 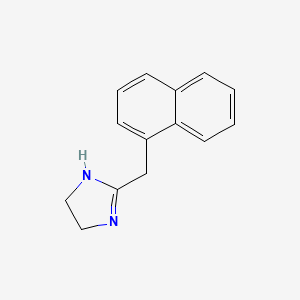 |
0.338 | ||
| ENC002926 | 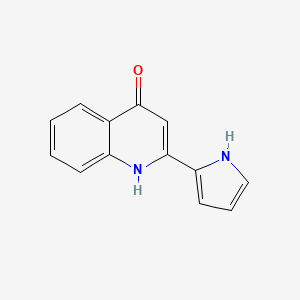 |
0.410 | D0K1XK | 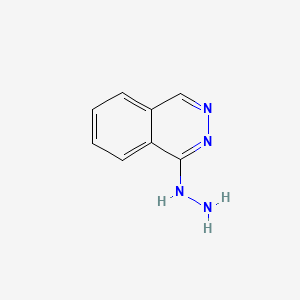 |
0.321 | ||
| ENC002154 | 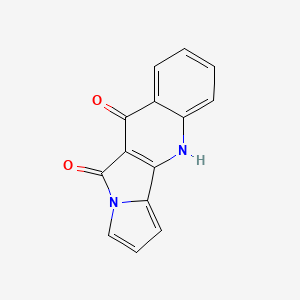 |
0.379 | D0IT2X | 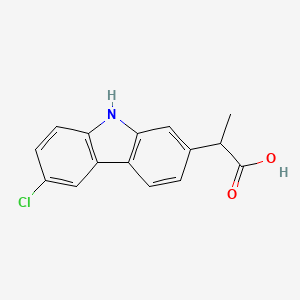 |
0.314 | ||
| ENC000041 |  |
0.375 | D0F5ZM | 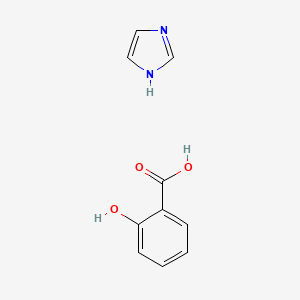 |
0.306 | ||
| ENC000036 | 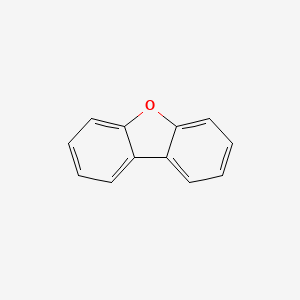 |
0.368 | D05EJG | 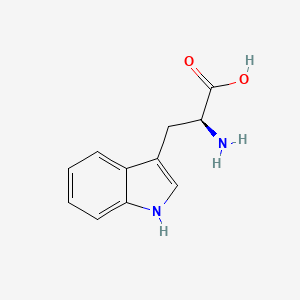 |
0.306 | ||
| ENC000858 | 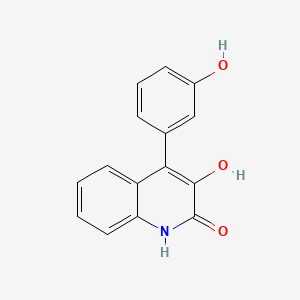 |
0.368 | D0B1FE | 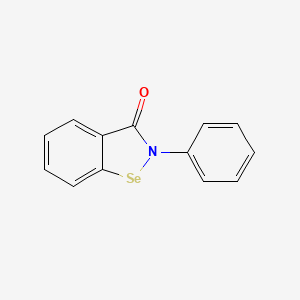 |
0.303 | ||
| ENC002699 | 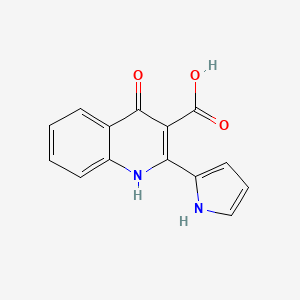 |
0.368 | D0QS1U | 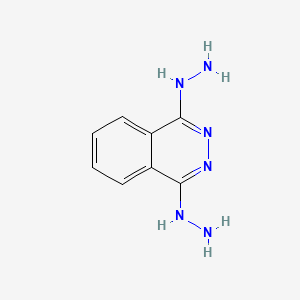 |
0.295 | ||
| ENC000341 |  |
0.365 | D00VUL | 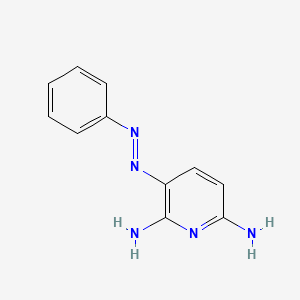 |
0.288 | ||
| ENC000167 | 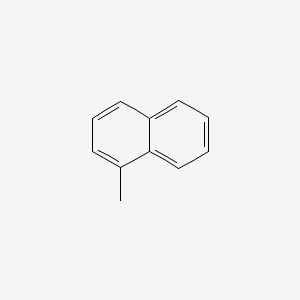 |
0.365 | D0E3SH | 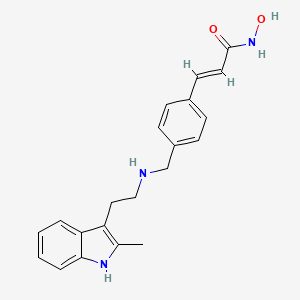 |
0.281 | ||