NPs Basic Information
|
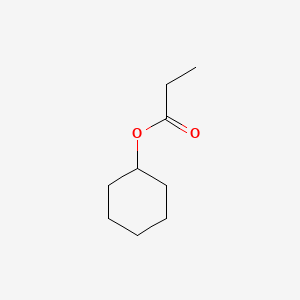
Name |
Cyclohexyl propionate
|
| Molecular Formula | C9H16O2 | |
| IUPAC Name* |
cyclohexyl propanoate
|
|
| SMILES |
CCC(=O)OC1CCCCC1
|
|
| InChI |
InChI=1S/C9H16O2/c1-2-9(10)11-8-6-4-3-5-7-8/h8H,2-7H2,1H3
|
|
| InChIKey |
MAMMVUWCKMOLSG-UHFFFAOYSA-N
|
|
| Synonyms |
CYCLOHEXYL PROPIONATE; 6222-35-1; Cyclohexyl propanoate; Propionic acid cyclohexyl ester; Propanoic acid, cyclohexyl ester; cyclohexylpropionate; Propionic acid, cyclohexyl ester; FEMA No. 2354; AI3-17708; Cyclohexyl n-propionate; 7697L79NDU; NSC-11770; UNII-7697L79NDU; EINECS 228-303-5; Cyclohexyl=propionate; starbld0016573; SCHEMBL183236; Cyclohexyl propionate, >=99%; FEMA 2354; DTXSID20211277; CHEBI:171769; NSC11770; ZINC1718570; CYCLOHEXYL PROPIONATE [FHFI]; MFCD00046352; NSC 11770; AKOS015961893; BS-23511; DB-054097; FT-0624199; P0504; D91966; 1H-Indazole-3-carboxylicacid, 6-bromo-, methyl ester; Q27266471
|
|
| CAS | 6222-35-1 | |
| PubChem CID | 61375 | |
| ChEMBL ID | NA |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 156.22 | ALogp: | 2.4 |
| HBD: | 0 | HBA: | 2 |
| Rotatable Bonds: | 3 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 26.3 | Aromatic Rings: | 1 |
| Heavy Atoms: | 11 | QED Weighted: | 0.574 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.407 | MDCK Permeability: | 0.00003810 |
| Pgp-inhibitor: | 0.303 | Pgp-substrate: | 0.008 |
| Human Intestinal Absorption (HIA): | 0.003 | 20% Bioavailability (F20%): | 0.039 |
| 30% Bioavailability (F30%): | 0.885 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.958 | Plasma Protein Binding (PPB): | 83.58% |
| Volume Distribution (VD): | 0.743 | Fu: | 20.34% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.412 | CYP1A2-substrate: | 0.166 |
| CYP2C19-inhibitor: | 0.172 | CYP2C19-substrate: | 0.136 |
| CYP2C9-inhibitor: | 0.105 | CYP2C9-substrate: | 0.473 |
| CYP2D6-inhibitor: | 0.011 | CYP2D6-substrate: | 0.113 |
| CYP3A4-inhibitor: | 0.105 | CYP3A4-substrate: | 0.22 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 9.601 | Half-life (T1/2): | 0.681 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.019 | Human Hepatotoxicity (H-HT): | 0.066 |
| Drug-inuced Liver Injury (DILI): | 0.193 | AMES Toxicity: | 0.008 |
| Rat Oral Acute Toxicity: | 0.021 | Maximum Recommended Daily Dose: | 0.153 |
| Skin Sensitization: | 0.915 | Carcinogencity: | 0.436 |
| Eye Corrosion: | 0.969 | Eye Irritation: | 0.982 |
| Respiratory Toxicity: | 0.201 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000492 | 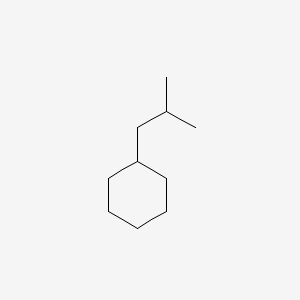 |
0.372 | D03DVJ | 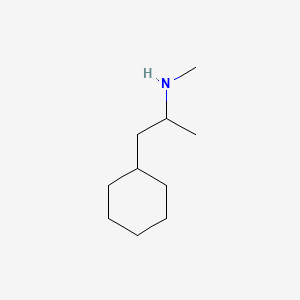 |
0.348 | ||
| ENC001283 |  |
0.362 | D04JPJ | 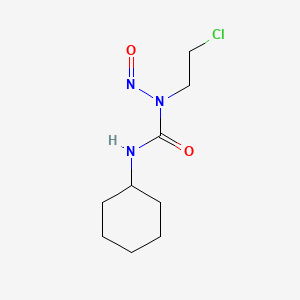 |
0.327 | ||
| ENC001306 | 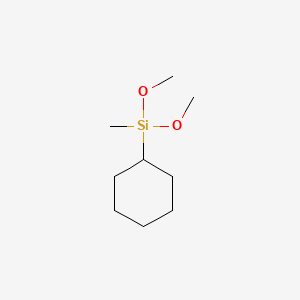 |
0.333 | D04URO | 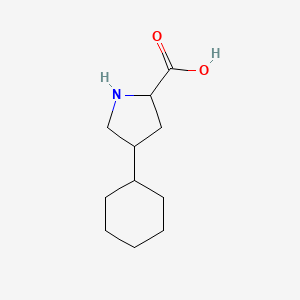 |
0.315 | ||
| ENC001222 | 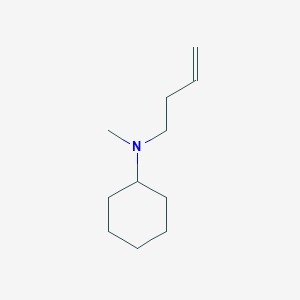 |
0.327 | D08MRN | 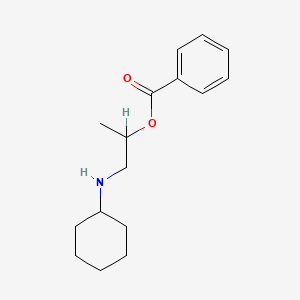 |
0.303 | ||
| ENC001169 | 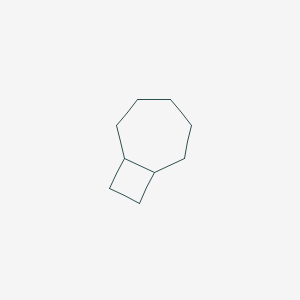 |
0.318 | D07XJM | 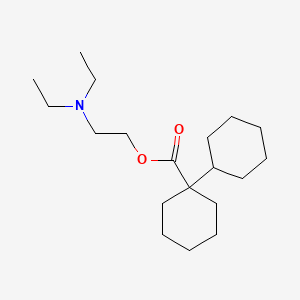 |
0.288 | ||
| ENC000183 | 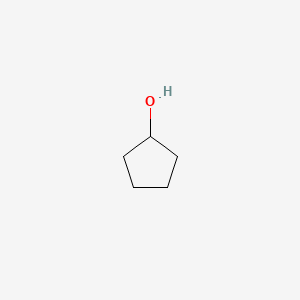 |
0.297 | D07GRH | 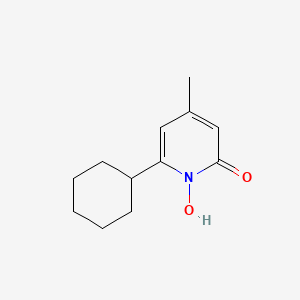 |
0.281 | ||
| ENC001167 | 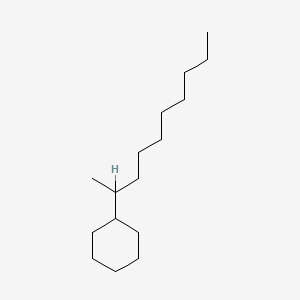 |
0.283 | D0R7WU | 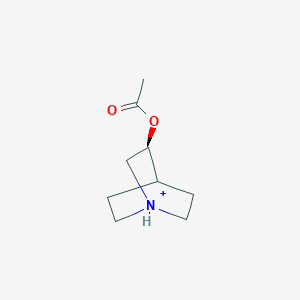 |
0.275 | ||
| ENC005521 | 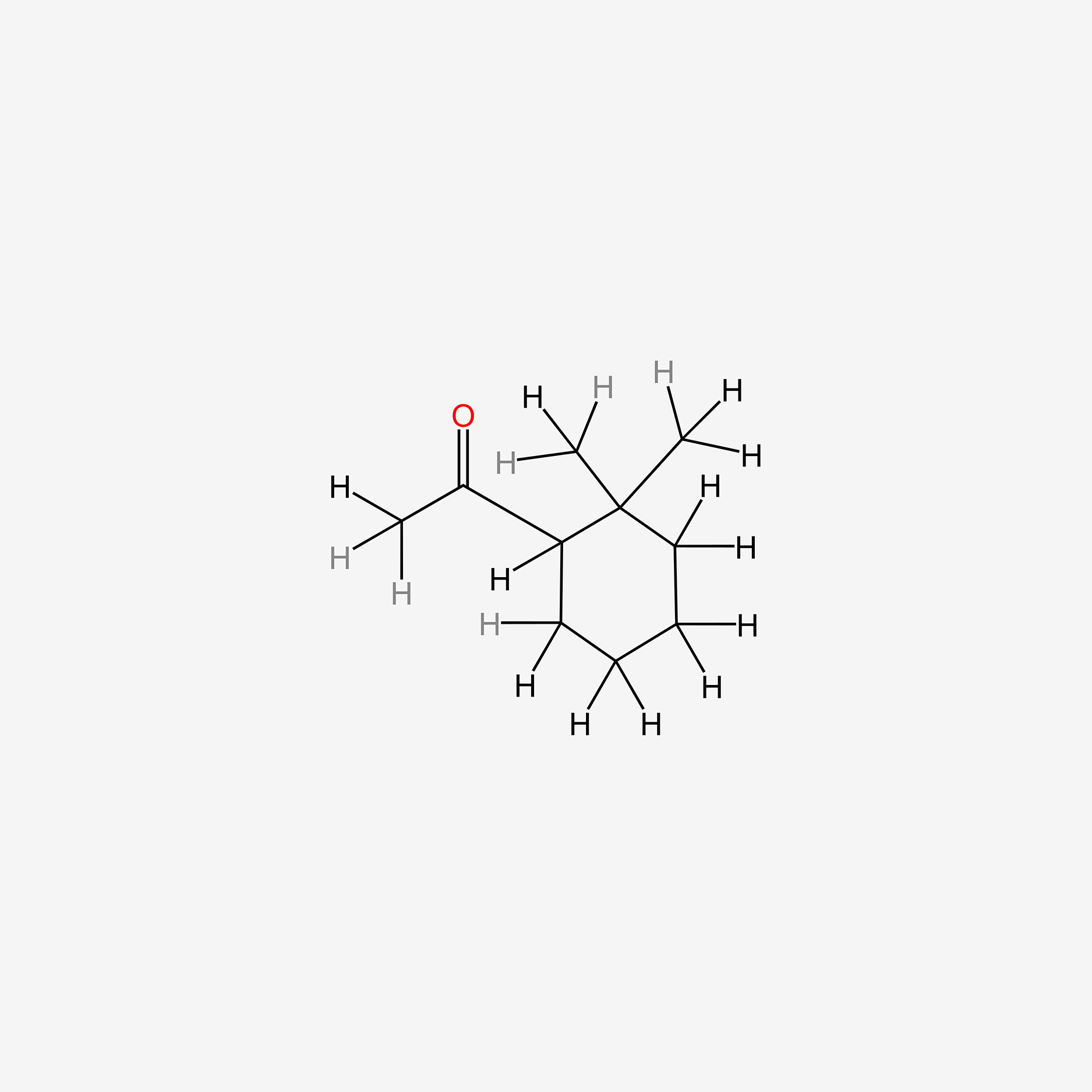 |
0.277 | D0Y3ME | 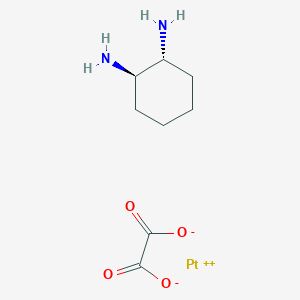 |
0.264 | ||
| ENC002063 | 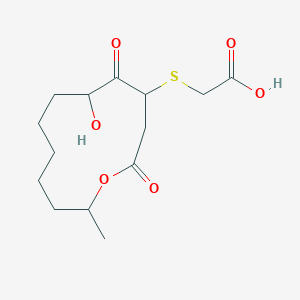 |
0.275 | D0D1MA | 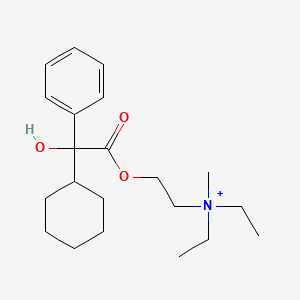 |
0.263 | ||
| ENC002200 | 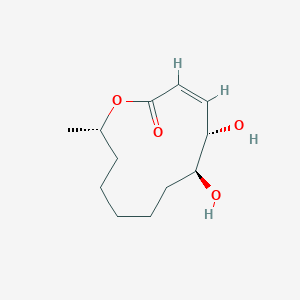 |
0.271 | D0J0ZS | 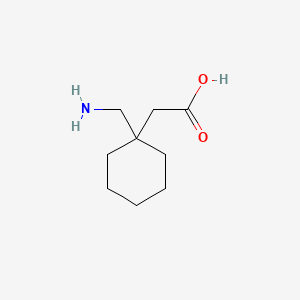 |
0.255 | ||