NPs Basic Information
|
Name |
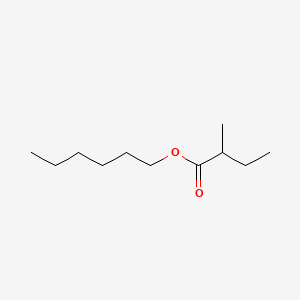
Hexyl 2-methylbutanoate
|
| Molecular Formula | C11H22O2 | |
| IUPAC Name* |
hexyl 2-methylbutanoate
|
|
| SMILES |
CCCCCCOC(=O)C(C)CC
|
|
| InChI |
InChI=1S/C11H22O2/c1-4-6-7-8-9-13-11(12)10(3)5-2/h10H,4-9H2,1-3H3
|
|
| InChIKey |
YUECNVSODFDKOQ-UHFFFAOYSA-N
|
|
| Synonyms |
Hexyl 2-methylbutanoate; Hexyl 2-methylbutyrate; 10032-15-2; Butanoic acid, 2-methyl-, hexyl ester; BUTYRIC ACID, 2-METHYL-, HEXYL ESTER; 2-Methylbutanoic acid, n-hexyl ester; FEMA No. 3499; HEXYL-2-METHYLBUTYRATE; hexyl 2-methyl butyrate; n-Hexyl 2-methylbutanoate; n-Hexyl 2-methyl butyrate; UI17LL5Q4P; exyl 2-methylbutanoate; Hexyl 2-methylbutanoate (natural); EINECS 233-106-2; UNII-UI17LL5Q4P; BRN 1856407; AI3-33623; Hexyl 2 Methyl Butyrate; Hexyl 2-methylbutanoate #; 2-Methylbutanoic acid hexyl; DSSTox_CID_27599; DSSTox_RID_82444; DSSTox_GSID_47599; Nat.Hexyl 2 Methyl Butyrate; SCHEMBL573736; CHEMBL3561573; DTXSID4047599; N-HEXYL 2-METHYLBUTYRATE; FEMA 3499; CHEBI:156491; Tox21_302599; LMFA07010906; HEXYL 2-METHYLBUTYRATE [FCC]; AKOS015903802; HEXYL 2-METHYLBUTYRATE [FHFI]; NCGC00256766-01; AS-76706; CAS-10032-15-2; CS-0439610; FT-0613028; H1788; Hexyl 2-methylbutanoate, >=95%, FCC, FG; Hexyl 2-methylbutanoate, analytical reference material; Hexyl 2-methylbutanoate, natural (US), >=95%, FG; Q27291087
|
|
| CAS | 10032-15-2 | |
| PubChem CID | 24838 | |
| ChEMBL ID | CHEMBL3561573 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 186.29 | ALogp: | 3.8 |
| HBD: | 0 | HBA: | 2 |
| Rotatable Bonds: | 8 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 26.3 | Aromatic Rings: | 0 |
| Heavy Atoms: | 13 | QED Weighted: | 0.444 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.326 | MDCK Permeability: | 0.00002300 |
| Pgp-inhibitor: | 0.023 | Pgp-substrate: | 0.001 |
| Human Intestinal Absorption (HIA): | 0.002 | 20% Bioavailability (F20%): | 0.986 |
| 30% Bioavailability (F30%): | 0.987 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.923 | Plasma Protein Binding (PPB): | 76.00% |
| Volume Distribution (VD): | 0.997 | Fu: | 29.24% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.969 | CYP1A2-substrate: | 0.758 |
| CYP2C19-inhibitor: | 0.772 | CYP2C19-substrate: | 0.698 |
| CYP2C9-inhibitor: | 0.51 | CYP2C9-substrate: | 0.44 |
| CYP2D6-inhibitor: | 0.042 | CYP2D6-substrate: | 0.133 |
| CYP3A4-inhibitor: | 0.173 | CYP3A4-substrate: | 0.239 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 9.903 | Half-life (T1/2): | 0.379 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.081 | Human Hepatotoxicity (H-HT): | 0.023 |
| Drug-inuced Liver Injury (DILI): | 0.092 | AMES Toxicity: | 0.006 |
| Rat Oral Acute Toxicity: | 0.065 | Maximum Recommended Daily Dose: | 0.012 |
| Skin Sensitization: | 0.755 | Carcinogencity: | 0.139 |
| Eye Corrosion: | 0.935 | Eye Irritation: | 0.984 |
| Respiratory Toxicity: | 0.607 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000726 |  |
0.641 | D0AY9Q |  |
0.426 | ||
| ENC000645 |  |
0.628 | D01QLH | 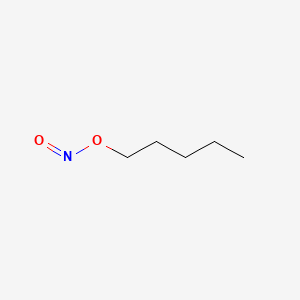 |
0.357 | ||
| ENC000655 | 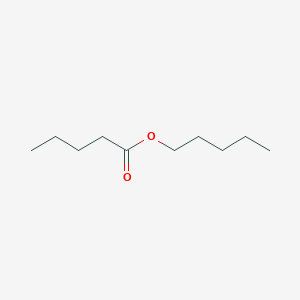 |
0.545 | D05ATI |  |
0.302 | ||
| ENC000188 | 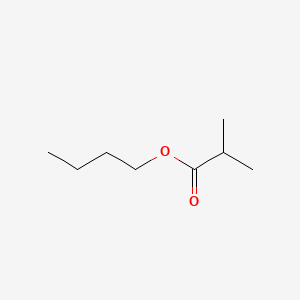 |
0.525 | D06ORU | 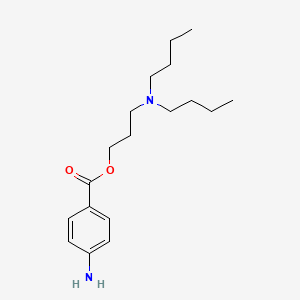 |
0.293 | ||
| ENC002444 | 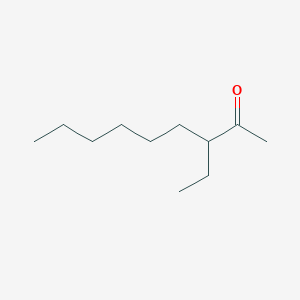 |
0.523 | D0ZK8H | 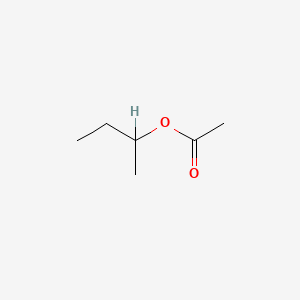 |
0.279 | ||
| ENC000554 | 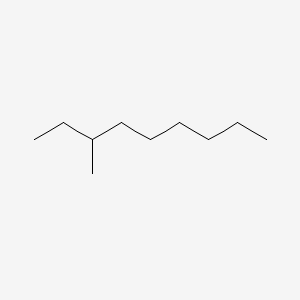 |
0.512 | D0Z5SM |  |
0.271 | ||
| ENC000742 | 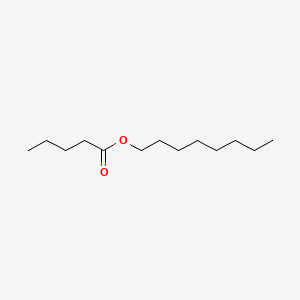 |
0.481 | D0Y3KG | 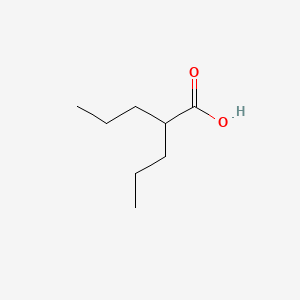 |
0.271 | ||
| ENC000797 | 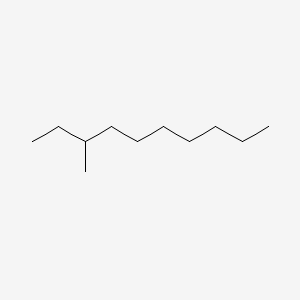 |
0.477 | D0X4FM | 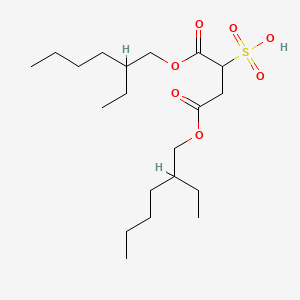 |
0.264 | ||
| ENC000855 | 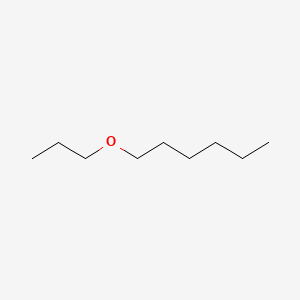 |
0.465 | D0H2SY | 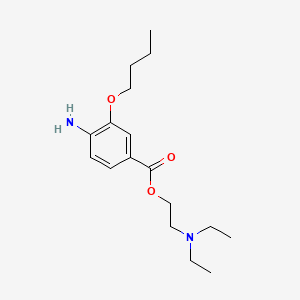 |
0.263 | ||
| ENC000228 | 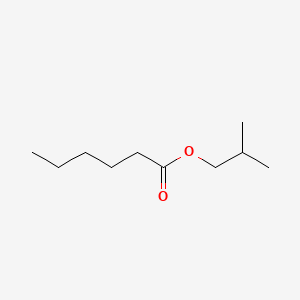 |
0.457 | D0G2KD | 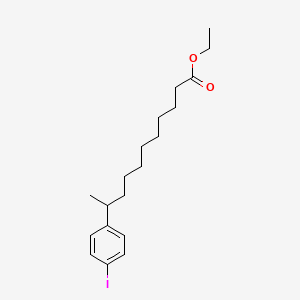 |
0.260 | ||