NPs Basic Information

|
Name |
Docosane
|
| Molecular Formula | C22H46 | |
| IUPAC Name* |
docosane
|
|
| SMILES |
CCCCCCCCCCCCCCCCCCCCCC
|
|
| InChI |
InChI=1S/C22H46/c1-3-5-7-9-11-13-15-17-19-21-22-20-18-16-14-12-10-8-6-4-2/h3-22H2,1-2H3
|
|
| InChIKey |
HOWGUJZVBDQJKV-UHFFFAOYSA-N
|
|
| Synonyms |
DOCOSANE; n-Docosane; 629-97-0; Heneicosane, methyl-; Dokosan; OW99Q363KO; CH3-[CH2]20-CH3; NSC-77139; Docosane, analytical standard; TWT; UNII-OW99Q363KO; Normal-docosane; Docosane, n-; EINECS 211-121-5; MFCD00009348; NSC 77139; Docosane, 99%; DOCOSANE [INCI]; PARAFOL 22-95; DTXSID7047063; CHEBI:46050; HSDB 8352; HY-N9929; NSC77139; ZINC6920415; LMFA11000569; STL453762; AKOS015901007; AS-56021; DB-054364; CS-0214009; D0962; FT-0625560; D95359; Q150968; ABD59C73-3CA6-4508-B950-18336DB59BE3
|
|
| CAS | 629-97-0 | |
| PubChem CID | 12405 | |
| ChEMBL ID | NA |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 310.6 | ALogp: | 11.5 |
| HBD: | 0 | HBA: | 0 |
| Rotatable Bonds: | 19 | Lipinski's rule of five: | Rejected |
| Polar Surface Area: | 0.0 | Aromatic Rings: | 0 |
| Heavy Atoms: | 22 | QED Weighted: | 0.187 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -5.105 | MDCK Permeability: | 0.00000541 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0 |
| Human Intestinal Absorption (HIA): | 0.003 | 20% Bioavailability (F20%): | 0.158 |
| 30% Bioavailability (F30%): | 1 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.021 | Plasma Protein Binding (PPB): | 98.54% |
| Volume Distribution (VD): | 4.533 | Fu: | 1.18% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.079 | CYP1A2-substrate: | 0.156 |
| CYP2C19-inhibitor: | 0.202 | CYP2C19-substrate: | 0.06 |
| CYP2C9-inhibitor: | 0.044 | CYP2C9-substrate: | 0.964 |
| CYP2D6-inhibitor: | 0.178 | CYP2D6-substrate: | 0.031 |
| CYP3A4-inhibitor: | 0.168 | CYP3A4-substrate: | 0.024 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 4.464 | Half-life (T1/2): | 0.022 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.353 | Human Hepatotoxicity (H-HT): | 0.005 |
| Drug-inuced Liver Injury (DILI): | 0.374 | AMES Toxicity: | 0.008 |
| Rat Oral Acute Toxicity: | 0.022 | Maximum Recommended Daily Dose: | 0.037 |
| Skin Sensitization: | 0.968 | Carcinogencity: | 0.023 |
| Eye Corrosion: | 0.996 | Eye Irritation: | 0.93 |
| Respiratory Toxicity: | 0.351 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000442 |  |
0.955 | D00AOJ |  |
0.819 | ||
| ENC000430 |  |
0.953 | D07ILQ | 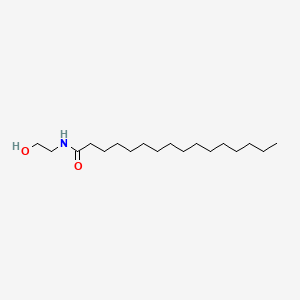 |
0.531 | ||
| ENC000446 |  |
0.914 | D00FGR |  |
0.511 | ||
| ENC000285 |  |
0.906 | D00STJ |  |
0.496 | ||
| ENC000433 |  |
0.877 | D0Z5SM |  |
0.475 | ||
| ENC000428 |  |
0.859 | D05ATI | 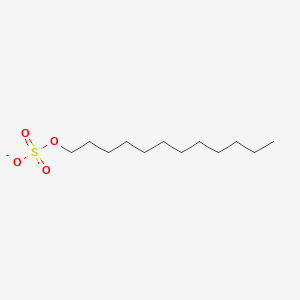 |
0.405 | ||
| ENC000761 |  |
0.855 | D0O1PH | 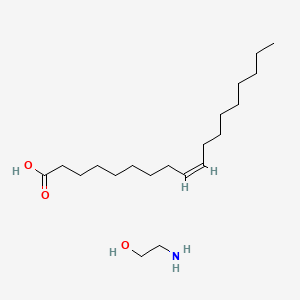 |
0.398 | ||
| ENC000434 |  |
0.842 | D0T9TJ |  |
0.385 | ||
| ENC000449 |  |
0.819 | D00MLW |  |
0.358 | ||
| ENC000750 |  |
0.819 | D0Z1QC |  |
0.330 | ||