NPs Basic Information
|
Name |
Chrysophanol
|
| Molecular Formula | C15H10O4 | |
| IUPAC Name* |
1,8-dihydroxy-3-methylanthracene-9,10-dione
|
|
| SMILES |
CC1=CC2=C(C(=C1)O)C(=O)C3=C(C2=O)C=CC=C3O
|
|
| InChI |
InChI=1S/C15H10O4/c1-7-5-9-13(11(17)6-7)15(19)12-8(14(9)18)3-2-4-10(12)16/h2-6,16-17H,1H3
|
|
| InChIKey |
LQGUBLBATBMXHT-UHFFFAOYSA-N
|
|
| Synonyms |
Chrysophanol; 481-74-3; CHRYSOPHANIC ACID; 1,8-Dihydroxy-3-methylanthraquinone; 3-Methylchrysazin; Turkey rhubarb; 1,8-dihydroxy-3-methylanthracene-9,10-dione; C.I. Natural Yellow 23; NSC 37132; 1,8-Dihydroxy-3-methyl-9,10-anthracenedione; NSC 646567; Crysophanic acid; 4,5-Dihydroxy-2-methylanthraquinone; Crysophanol; 2-Methyl-4,5-dihydroxyanthraquinone; 3-Methyl-1,8-dihydroxyanthraquinone; C.I. 75400; 1,8-Dihydroxy-3-Methyl-Anthraquinone; CHEBI:3687; NSC-37132; NSC-646567; N1ST8V8RR2; Anthraquinone, 1,8-dihydroxy-3-methyl-; Chrysophanic acid (1,8-dihydroxy-3-methylanthraquinone); 1,8-dihydroxy-3-methyl-9,10-anthraquinone; 1,8-Dihydroxy-3-methylanthra-9,10-quinone; 9,10-Anthracenedione, 1,8-dihydroxy-3-methyl-; NSC646567; CCRIS 3525; EINECS 207-572-2; UNII-N1ST8V8RR2; Archinin; Rumicin; Chrysophansaeure; MFCD00001208; Spectrum_000792; Chrysophanic Acid,(S); SpecPlus_000321; Spectrum2_000043; Spectrum3_001183; Spectrum4_001477; Spectrum5_000153; DSSTox_CID_4832; 1,8-dihydroxy-3-methyl-anthracene-9,10-dione; DSSTox_RID_77547; DSSTox_GSID_24832; BSPBio_002825; KBioGR_002053; KBioSS_001272; SPECTRUM300545; MLS000574888; CHEMBL41092; DivK1c_006417; SCHEMBL308131; SPBio_000165; CHRYSOPHANIC ACID [MI]; 9, 1,8-dihydroxy-3-methyl-; Chrysophanic acid (Chrysophanol); Chrysophanic acid - Chrysophanol; DTXSID6024832; Chrysophanol, analytical standard; HSDB 8483; KBio1_001361; KBio2_001272; KBio2_003840; KBio2_006408; KBio3_002325; HMS3656G17; CHRYSOPHANIC ACID [WHO-DD]; BCP23439; EX-A6777; NSC37132; ZINC3861630; Tox21_202499; Anthraquinone,8-dihydroxy-3-methyl-; BDBM50455992; CCG-38348; LMPK13040006; s2406; STL564344; 3-Methyl-1, 8-dihydroxyanthraquinone; 4, 5-Dihydroxy-2-methylanthraquinone; AKOS015905000; AC-7980; BCP9000004; DS-9706; SDCCGMLS-0066504.P001; NCGC00091823-01; NCGC00091823-02; NCGC00091823-03; NCGC00091823-04; NCGC00091823-05; NCGC00260048-01; CAS-481-74-3; HY-13595; SMR000156239; 1,8-Dihydroxy-3-methylanthraquinone, 98%; 8,9-dihydroxy-6-methyl-1,10-anthraquinone; FT-0623813; SW219732-1; 1,8-Dihydroxy-3-methylanthra-9,10-quinone #; EN300-7401332; 481C743; A827496; N-(diaminomethylene)formamide;3-METHYLCHRYSAZIN; SR-01000712223; 1,8-Dihydroxy-3-methyl-9,10-anthracenedione, 9CI; Q-100527; SR-01000712223-2; BRD-K59284035-001-02-0; BRD-K59284035-001-03-8; Q15410875; 9,10-Anthracenedione, 1,8-dihydroxy-3-methyl- (9CI); 1,8-DIHYDROXY-3-METHYL-9,10-DIHYDROANTHRACENE-9,10-DIONE
|
|
| CAS | 481-74-3 | |
| PubChem CID | 10208 | |
| ChEMBL ID | CHEMBL41092 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 254.24 | ALogp: | 3.5 |
| HBD: | 2 | HBA: | 4 |
| Rotatable Bonds: | 0 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 74.6 | Aromatic Rings: | 3 |
| Heavy Atoms: | 19 | QED Weighted: | 0.646 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.936 | MDCK Permeability: | 0.00001400 |
| Pgp-inhibitor: | 0.034 | Pgp-substrate: | 0.001 |
| Human Intestinal Absorption (HIA): | 0.009 | 20% Bioavailability (F20%): | 0.064 |
| 30% Bioavailability (F30%): | 0.998 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.135 | Plasma Protein Binding (PPB): | 99.64% |
| Volume Distribution (VD): | 0.457 | Fu: | 0.83% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.954 | CYP1A2-substrate: | 0.411 |
| CYP2C19-inhibitor: | 0.279 | CYP2C19-substrate: | 0.06 |
| CYP2C9-inhibitor: | 0.636 | CYP2C9-substrate: | 0.402 |
| CYP2D6-inhibitor: | 0.537 | CYP2D6-substrate: | 0.228 |
| CYP3A4-inhibitor: | 0.599 | CYP3A4-substrate: | 0.138 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 6.205 | Half-life (T1/2): | 0.137 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.027 | Human Hepatotoxicity (H-HT): | 0.046 |
| Drug-inuced Liver Injury (DILI): | 0.942 | AMES Toxicity: | 0.886 |
| Rat Oral Acute Toxicity: | 0.23 | Maximum Recommended Daily Dose: | 0.807 |
| Skin Sensitization: | 0.517 | Carcinogencity: | 0.908 |
| Eye Corrosion: | 0.006 | Eye Irritation: | 0.98 |
| Respiratory Toxicity: | 0.072 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
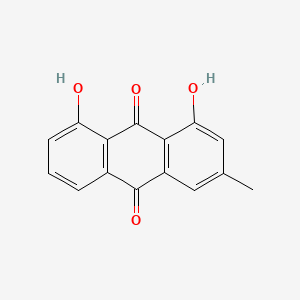
| ENC000094 | 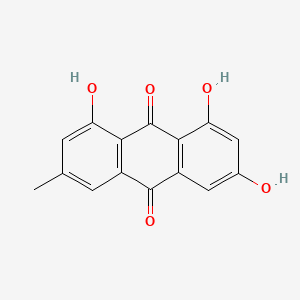 |
0.683 | D0N1FS | 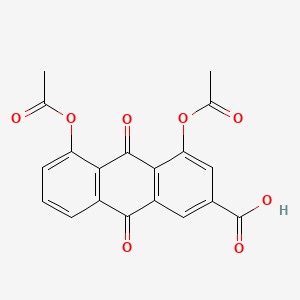 |
0.420 | ||
| ENC004888 | 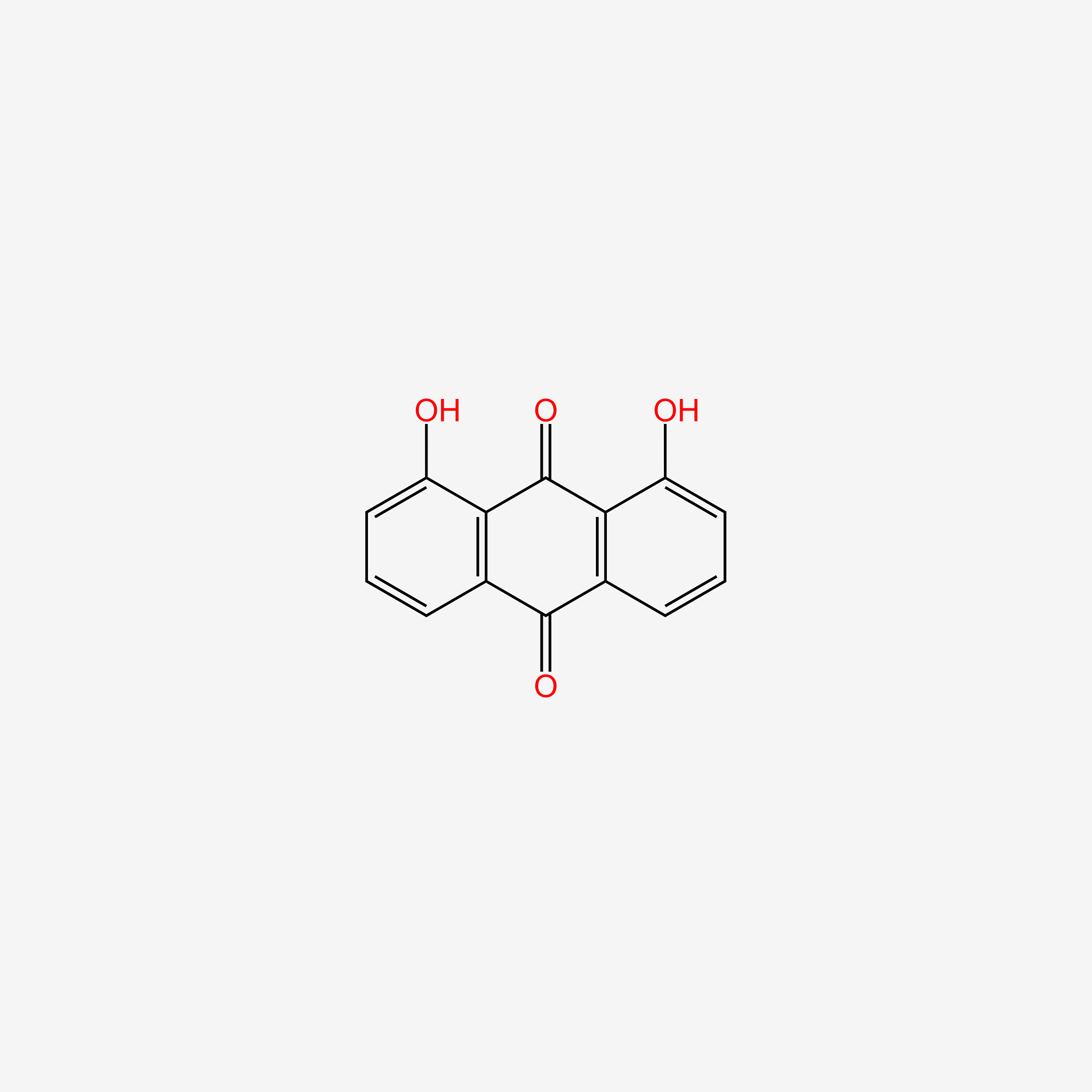 |
0.672 | D03GET | 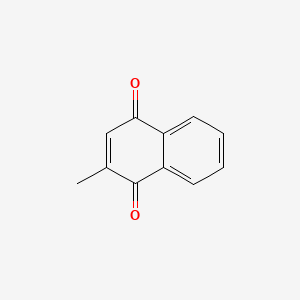 |
0.354 | ||
| ENC000087 | 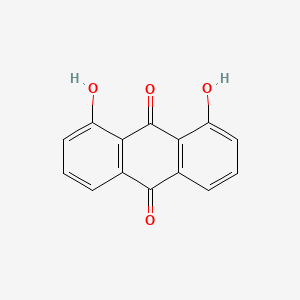 |
0.672 | D07MGA |  |
0.329 | ||
| ENC000362 | 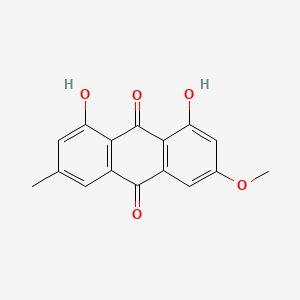 |
0.652 | D04AIT |  |
0.310 | ||
| ENC000939 | 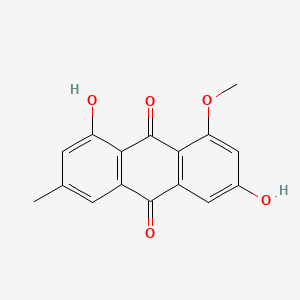 |
0.603 | D0Y7PG | 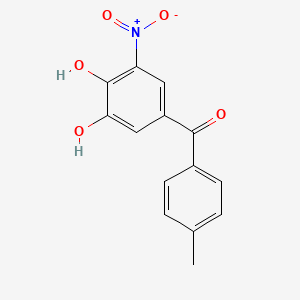 |
0.309 | ||
| ENC002031 | 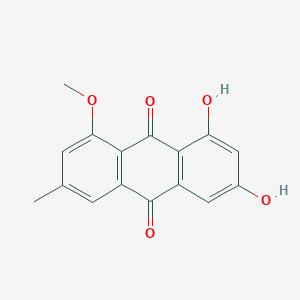 |
0.580 | D0K8KX |  |
0.302 | ||
| ENC002125 | 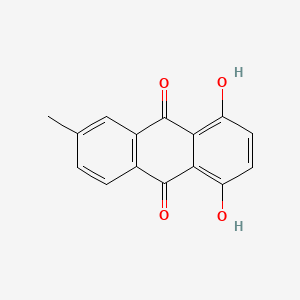 |
0.576 | D0H6QU | 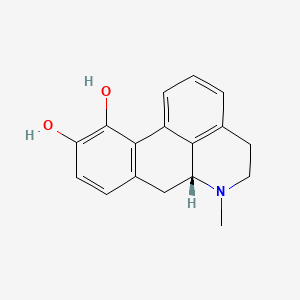 |
0.298 | ||
| ENC005490 | 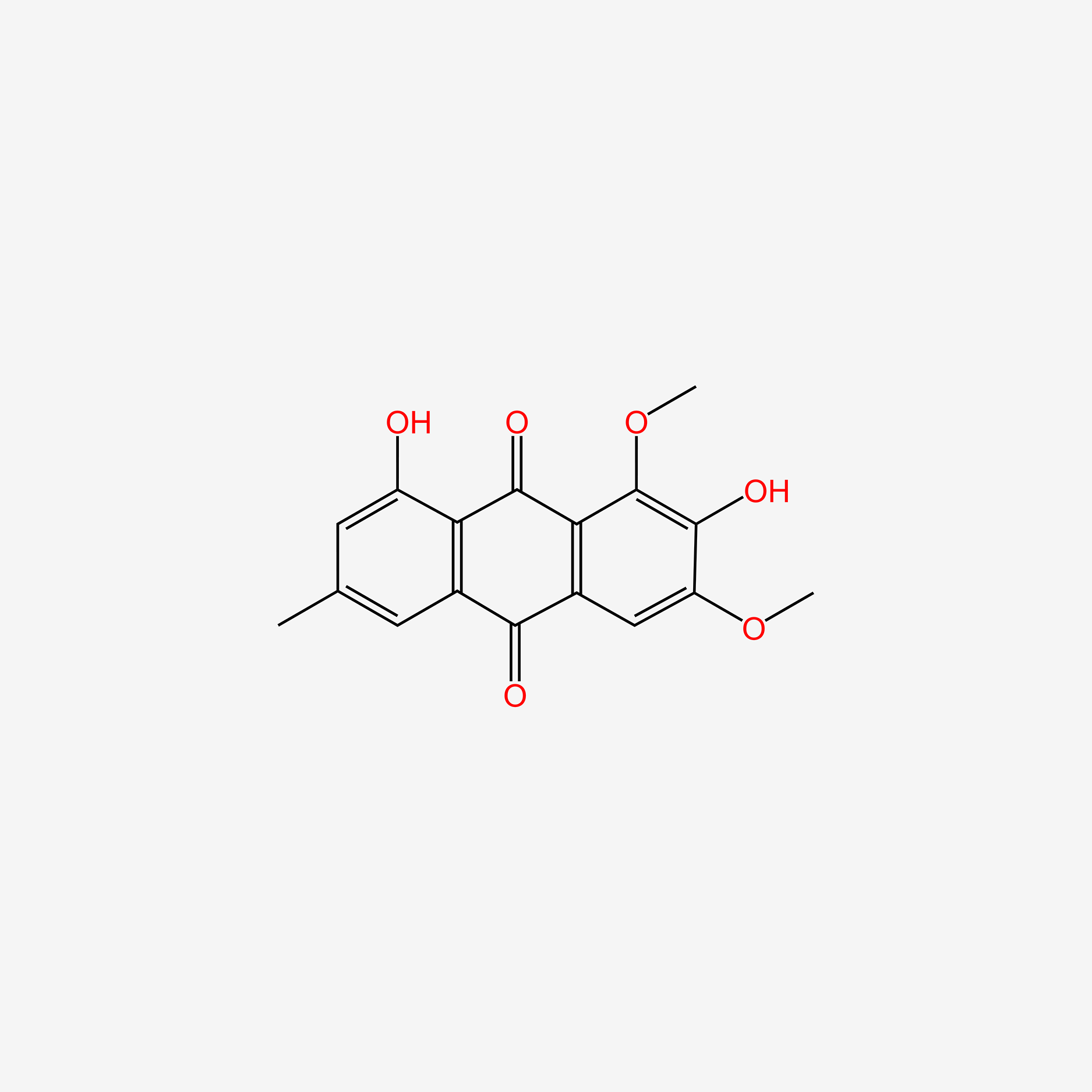 |
0.541 | D0H1AR | 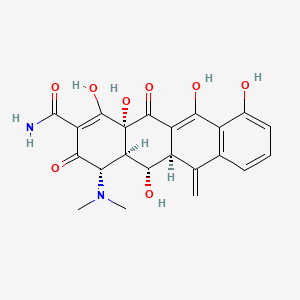 |
0.295 | ||
| ENC005573 | 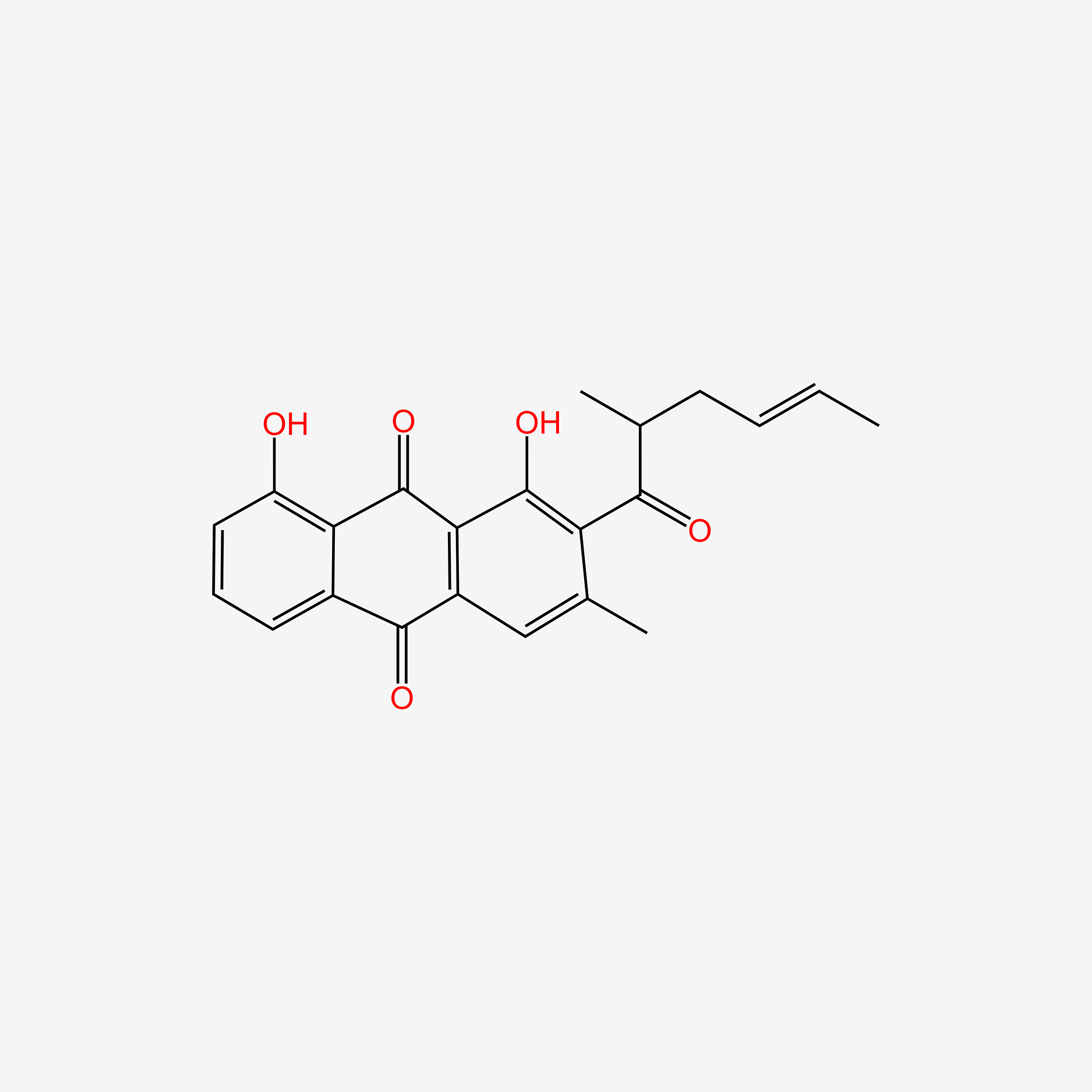 |
0.524 | D0H2ZW | 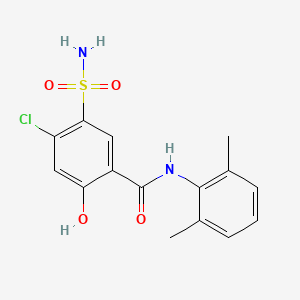 |
0.284 | ||
| ENC005572 | 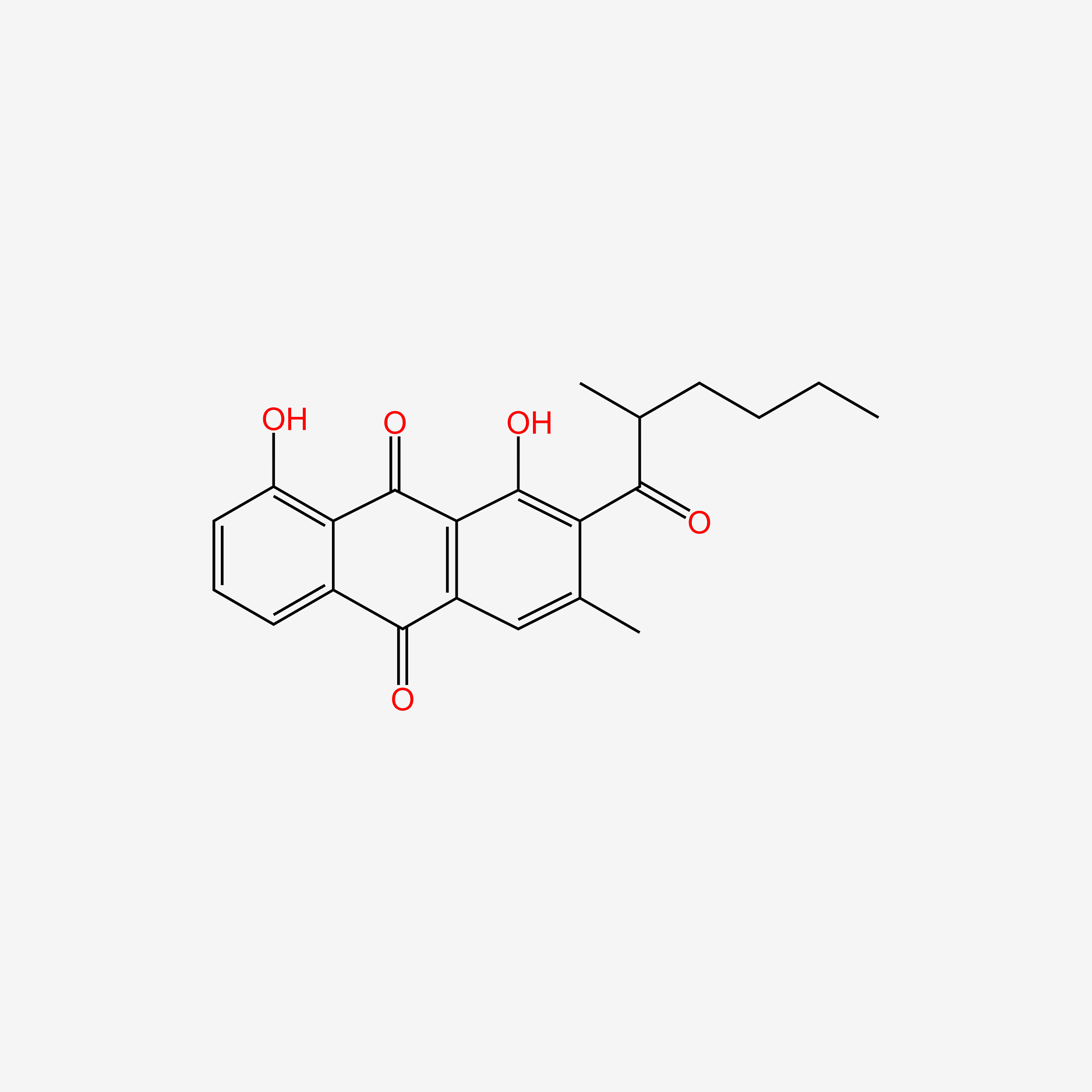 |
0.524 | D0R3JB | 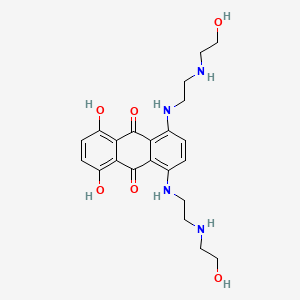 |
0.279 | ||