NPs Basic Information
|
Name |
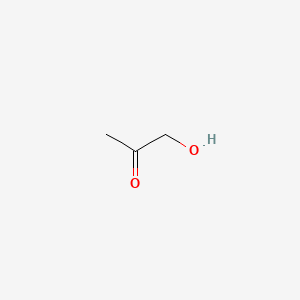
Hydroxyacetone
|
| Molecular Formula | C3H6O2 | |
| IUPAC Name* |
1-hydroxypropan-2-one
|
|
| SMILES |
CC(=O)CO
|
|
| InChI |
InChI=1S/C3H6O2/c1-3(5)2-4/h4H,2H2,1H3
|
|
| InChIKey |
XLSMFKSTNGKWQX-UHFFFAOYSA-N
|
|
| Synonyms |
Hydroxyacetone; 116-09-6; Acetol; 1-hydroxypropan-2-one; Acetone alcohol; 1-Hydroxy-2-propanone; 2-Propanone, 1-hydroxy-; 1-Hydroxyacetone; Acetylmethanol; ACETYLCARBINOL; Methanol, acetyl-; 2-Oxopropanol; Hydroxypropanone; Hydroxy-2-propanone; Methylketol; Pyruvinalcohol; Pyruvic alcohol; monohydroxyacetone; 2-Ketopropyl alcohol; 1-hydroxy-propan-2-one; 7I7YM0835W; MFCD00004669; NSC-102497; Hydroxymethyl methyl ketone; EINECS 204-124-8; NSC 102497; BRN 0605368; hydroxy acetone; hydroxy-acetone; UNII-7I7YM0835W; hydroxyl acetone; hydroxyl-acetone; AI3-37788; 2-proponol; 2-oxopropyl alcohol; hydroxypropan-2-one; 1-Hydroxyacetone #; ACETOMETHANOL; 1-Hydroxy-2-acetone; 1-oxidanylpropan-2-one; CH3COCH2OH; ACETOL [MI]; CH3C(O)CH2OH; WLN: Q1V1; Hydroxyacetone, >=95%, FG; DTXSID8051590; FEMA NO. 4462; CHEBI:27957; XLSMFKSTNGKWQX-UHFFFAOYSA-; ZINC895664; HY-Y1366; BBL011436; NSC102497; STL146544; AKOS000269101; s12357; DB-003685; CS-0017823; FT-0627141; H0388; EN300-20541; C05235; A803556; J-003383; Q20236702; F0001-0286; Hydroxyacetone contains =500 ppm sodium carbonate as stabilizer; Hydroxyacetone, contains <=500 ppm sodium carbonate as stabilizer, technical grade, 90%
|
|
| CAS | 116-09-6 | |
| PubChem CID | 8299 | |
| ChEMBL ID | NA |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 74.08 | ALogp: | -0.7 |
| HBD: | 1 | HBA: | 2 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 37.3 | Aromatic Rings: | 0 |
| Heavy Atoms: | 5 | QED Weighted: | 0.475 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.509 | MDCK Permeability: | 0.00012584 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.053 |
| Human Intestinal Absorption (HIA): | 0.005 | 20% Bioavailability (F20%): | 0.005 |
| 30% Bioavailability (F30%): | 0.003 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.566 | Plasma Protein Binding (PPB): | 15.24% |
| Volume Distribution (VD): | 0.612 | Fu: | 86.28% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.075 | CYP1A2-substrate: | 0.357 |
| CYP2C19-inhibitor: | 0.023 | CYP2C19-substrate: | 0.483 |
| CYP2C9-inhibitor: | 0.005 | CYP2C9-substrate: | 0.173 |
| CYP2D6-inhibitor: | 0.004 | CYP2D6-substrate: | 0.362 |
| CYP3A4-inhibitor: | 0.005 | CYP3A4-substrate: | 0.186 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 5.39 | Half-life (T1/2): | 0.911 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.025 | Human Hepatotoxicity (H-HT): | 0.035 |
| Drug-inuced Liver Injury (DILI): | 0.176 | AMES Toxicity: | 0.036 |
| Rat Oral Acute Toxicity: | 0.344 | Maximum Recommended Daily Dose: | 0.037 |
| Skin Sensitization: | 0.698 | Carcinogencity: | 0.036 |
| Eye Corrosion: | 0.978 | Eye Irritation: | 0.994 |
| Respiratory Toxicity: | 0.162 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000009 | 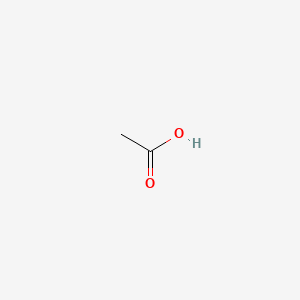 |
0.462 | D04CRL | 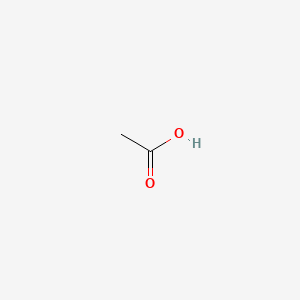 |
0.462 | ||
| ENC000377 | 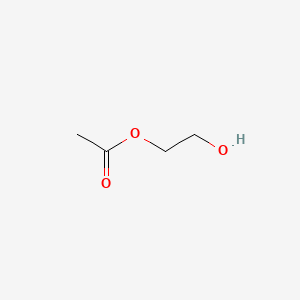 |
0.381 | D0R9BG | 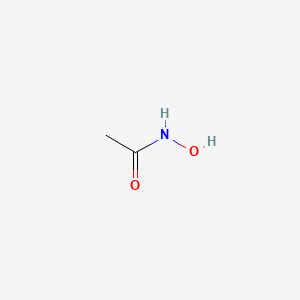 |
0.375 | ||
| ENC000058 | 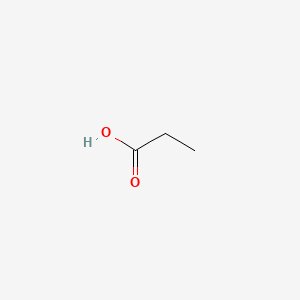 |
0.375 | D09KDV | 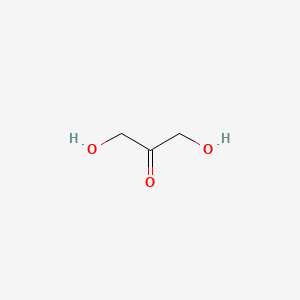 |
0.368 | ||
| ENC000008 | 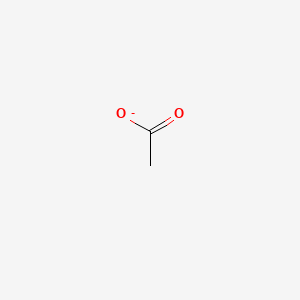 |
0.357 | D0C1PY | 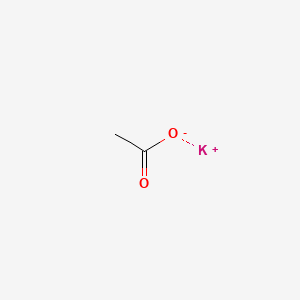 |
0.333 | ||
| ENC000237 | 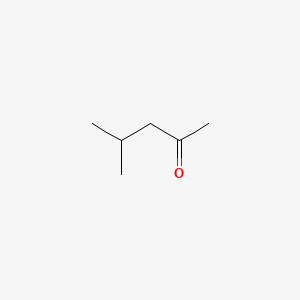 |
0.333 | D0Z4UY | 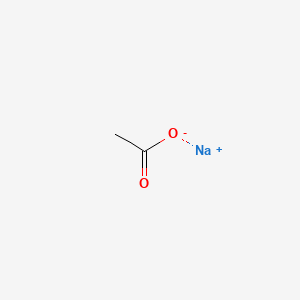 |
0.333 | ||
| ENC000061 | 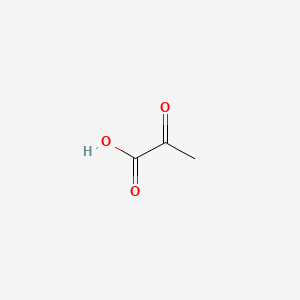 |
0.333 | D0G4JI | 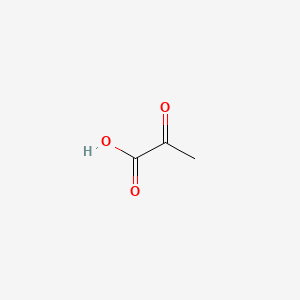 |
0.333 | ||
| ENC000010 | 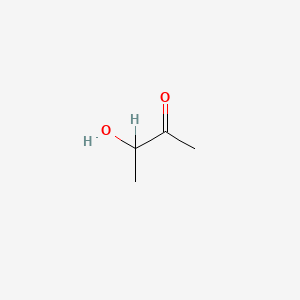 |
0.333 | D00AMQ | 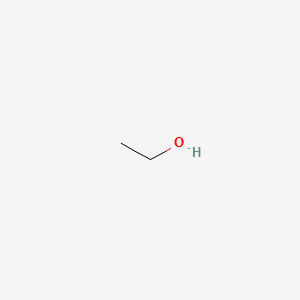 |
0.308 | ||
| ENC000532 | 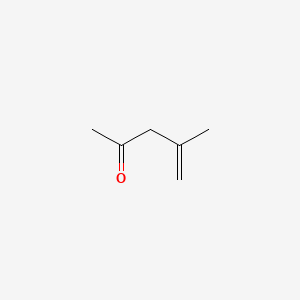 |
0.333 | D0M8AB | 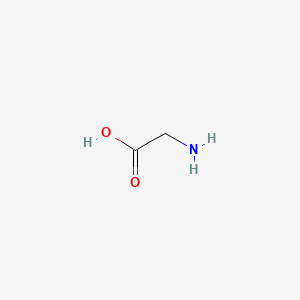 |
0.294 | ||
| ENC005511 |  |
0.308 | D02UDJ | 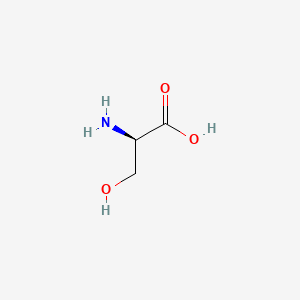 |
0.273 | ||
| ENC000039 | 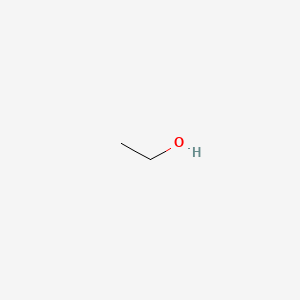 |
0.308 | D09PUL | 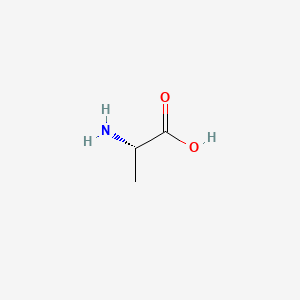 |
0.263 | ||