NPs Basic Information
|
Name |
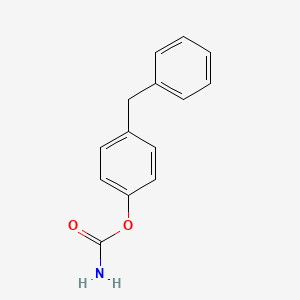
Diphenan
|
| Molecular Formula | C14H13NO2 | |
| IUPAC Name* |
(4-benzylphenyl) carbamate
|
|
| SMILES |
C1=CC=C(C=C1)CC2=CC=C(C=C2)OC(=O)N
|
|
| InChI |
InChI=1S/C14H13NO2/c15-14(16)17-13-8-6-12(7-9-13)10-11-4-2-1-3-5-11/h1-9H,10H2,(H2,15,16)
|
|
| InChIKey |
ZBJBRUSGEJORQL-UHFFFAOYSA-N
|
|
| Synonyms |
Diphenan; Carbaurine; Carphenol; Diphenane; Parabencil; Butolan; Butolen; Oxybulan; Palafuge; 101-71-3; p-Benzylphenyl carbamate; (4-benzylphenyl) carbamate; (4-benzylphenyl)carbamate; GNF-Pf-1544; .alpha.-Phenyl-p-cresol carbamate; Diphenan, pharmaceutical; 4-Benzylphenyl carbamate; NSC-60023; Phenol, 4-(phenylmethyl)-, carbamate; U129BBY8DB; NCGC00160564-01; p-Hydroxydiphenylmethane carbamic acid ester; Carbamic acid, .alpha.-phenyl-p-tolyl ester; Parabencilfenol; Diphenanum; Difenano; Diphenan [INN:DCF]; Difenano [INN-Spanish]; Diphenane [INN-French]; Diphenanum [INN-Latin]; Diphenan (pharmaceutical); Parabencilfenol [Spanish]; 4-(Phenylmethyl)phenol carbamate; p-Cresol, alpha-phenyl-, carbamate; UNII-U129BBY8DB; BRN 3295222; alpha-Phenyl-p-cresol carbamate; Phenol, carbamate; p-Cresol, carbamate; DIPHENAN [INN]; DIPHENANE [MI]; DSSTox_CID_26234; DSSTox_RID_81461; DSSTox_GSID_46234; 3-06-00-03360 (Beilstein Handbook Reference); SCHEMBL667724; CHEMBL608856; ZINC1314; DTXSID4046234; CHEBI:134933; NSC60023; Tox21_111903; NSC 60023; AKOS024332138; p-Cresol, .alpha.-phenyl-, carbamate; CAS-101-71-3; SR-01000945032; SR-01000945032-1; Q27290543
|
|
| CAS | 101-71-3 | |
| PubChem CID | 7572 | |
| ChEMBL ID | CHEMBL608856 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 227.26 | ALogp: | 3.0 |
| HBD: | 1 | HBA: | 2 |
| Rotatable Bonds: | 4 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 52.3 | Aromatic Rings: | 2 |
| Heavy Atoms: | 17 | QED Weighted: | 0.872 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.61 | MDCK Permeability: | 0.00003550 |
| Pgp-inhibitor: | 0.009 | Pgp-substrate: | 0.007 |
| Human Intestinal Absorption (HIA): | 0.007 | 20% Bioavailability (F20%): | 0.073 |
| 30% Bioavailability (F30%): | 0.057 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.916 | Plasma Protein Binding (PPB): | 95.02% |
| Volume Distribution (VD): | 0.48 | Fu: | 4.38% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.606 | CYP1A2-substrate: | 0.124 |
| CYP2C19-inhibitor: | 0.733 | CYP2C19-substrate: | 0.188 |
| CYP2C9-inhibitor: | 0.607 | CYP2C9-substrate: | 0.263 |
| CYP2D6-inhibitor: | 0.033 | CYP2D6-substrate: | 0.291 |
| CYP3A4-inhibitor: | 0.151 | CYP3A4-substrate: | 0.659 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 12.496 | Half-life (T1/2): | 0.477 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.15 | Human Hepatotoxicity (H-HT): | 0.438 |
| Drug-inuced Liver Injury (DILI): | 0.688 | AMES Toxicity: | 0.942 |
| Rat Oral Acute Toxicity: | 0.047 | Maximum Recommended Daily Dose: | 0.831 |
| Skin Sensitization: | 0.727 | Carcinogencity: | 0.853 |
| Eye Corrosion: | 0.003 | Eye Irritation: | 0.167 |
| Respiratory Toxicity: | 0.025 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000908 | 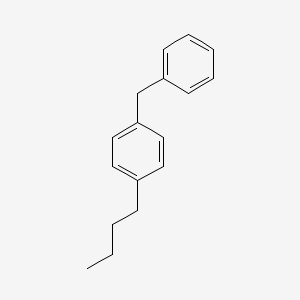 |
0.547 | D0H6TP | 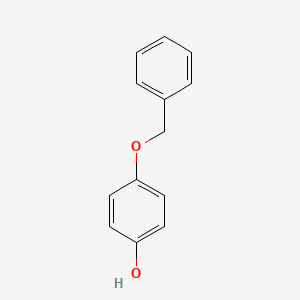 |
0.525 | ||
| ENC001400 | 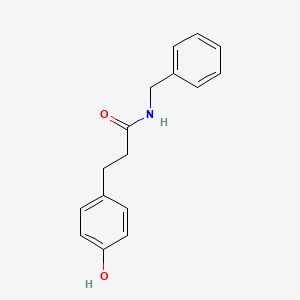 |
0.486 | D0Y7EM | 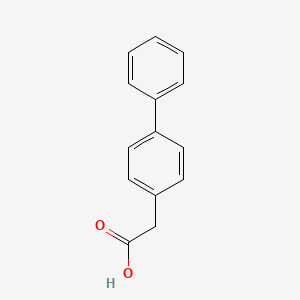 |
0.484 | ||
| ENC000302 | 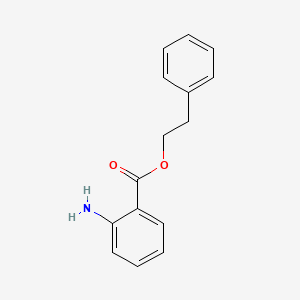 |
0.443 | D0G1VX |  |
0.433 | ||
| ENC000077 | 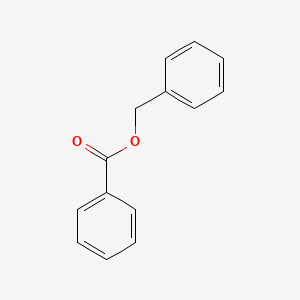 |
0.433 | D0L5PO | 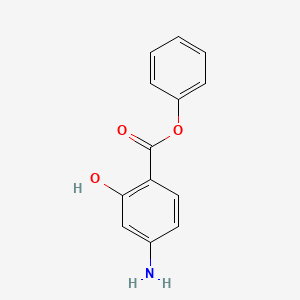 |
0.426 | ||
| ENC005617 | 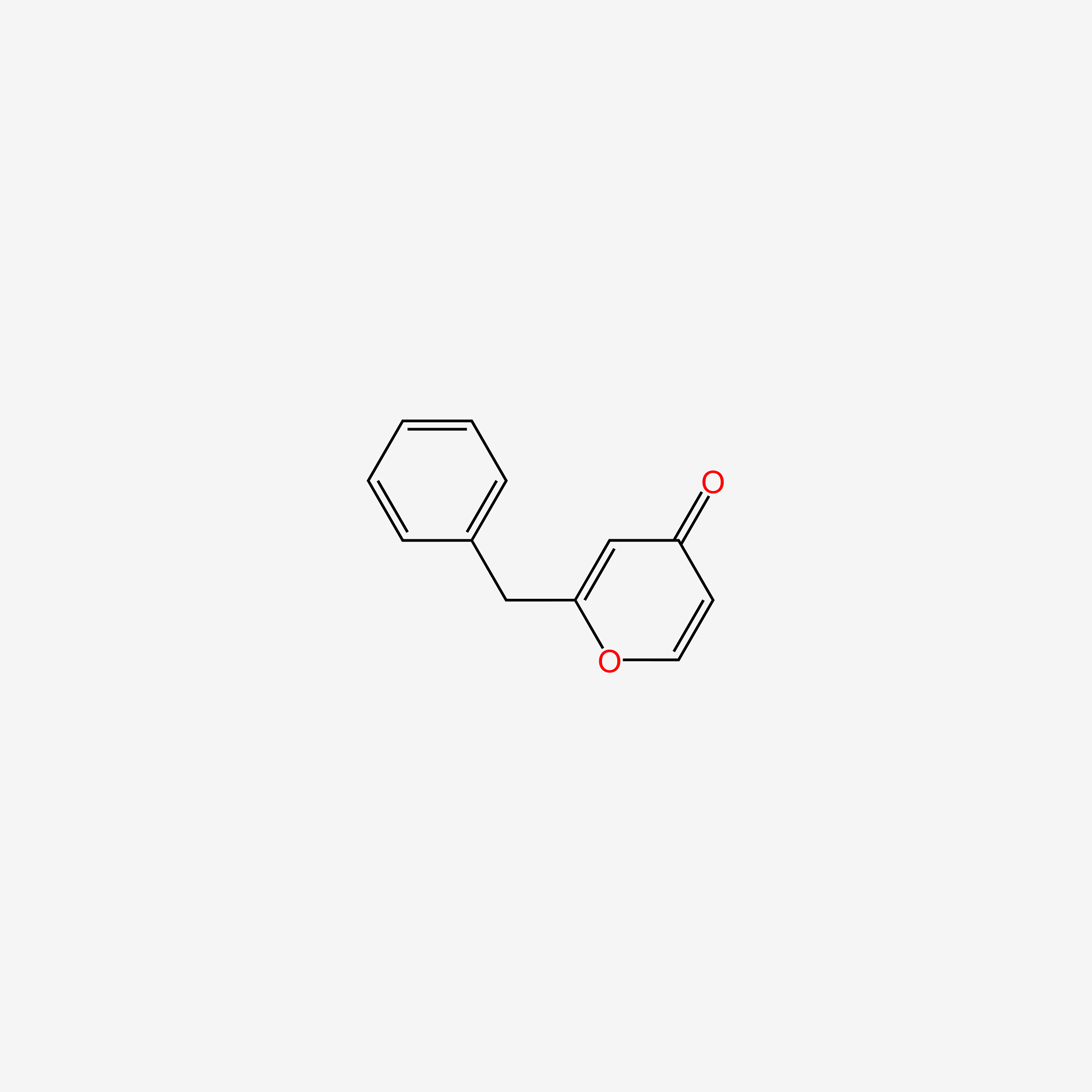 |
0.429 | D0TV6I | 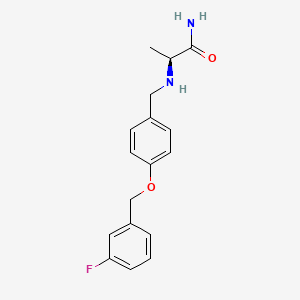 |
0.423 | ||
| ENC000219 | 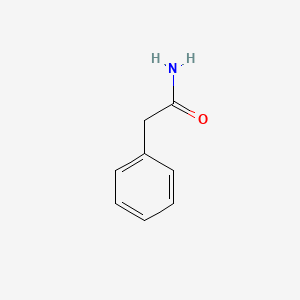 |
0.426 | D0KS6W | 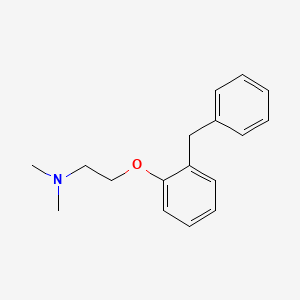 |
0.387 | ||
| ENC005854 |  |
0.426 | D06LHG | 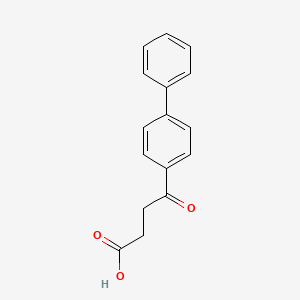 |
0.373 | ||
| ENC001456 | 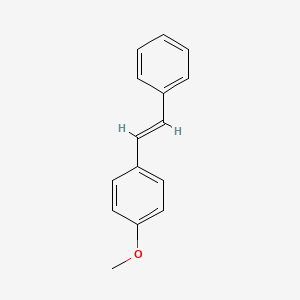 |
0.412 | D07ONP | 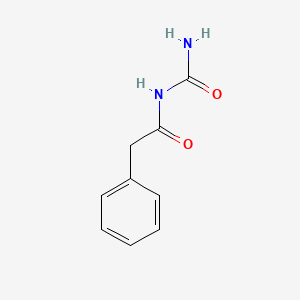 |
0.371 | ||
| ENC001523 | 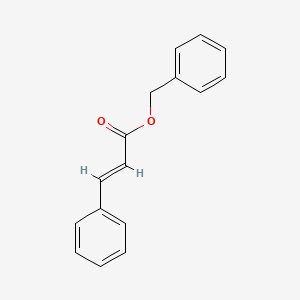 |
0.397 | D03XYW |  |
0.370 | ||
| ENC001805 |  |
0.392 | D0R1CR |  |
0.367 | ||