NPs Basic Information
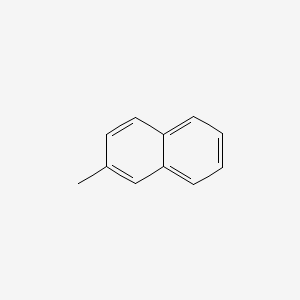
|
Name |
2-Methylnaphthalene
|
| Molecular Formula | C11H10 | |
| IUPAC Name* |
2-methylnaphthalene
|
|
| SMILES |
CC1=CC2=CC=CC=C2C=C1
|
|
| InChI |
InChI=1S/C11H10/c1-9-6-7-10-4-2-3-5-11(10)8-9/h2-8H,1H3
|
|
| InChIKey |
QIMMUPPBPVKWKM-UHFFFAOYSA-N
|
|
| Synonyms |
2-METHYLNAPHTHALENE; 91-57-6; Naphthalene, 2-methyl-; beta-Methylnaphthalene; .beta.-Methylnaphthalene; Naphthalene, beta-methyl-; 2-methyl-naphthalene; HSDB 5274; beta-methyl naphthalenes; S8MCX3C16H; CHEMBL195895; CHEBI:50720; NSC-3575; NAPHTALENE,2-METHYL MFC11 H10; 2-Methylnaphthalene, analytical standard; 2-Naphthylmethyl radical; NSC 3575; EINECS 202-078-3; UNII-S8MCX3C16H; AI3-17554; 2-methyInaphthalene; 2-methyl naphthalene; MFCD00004118; Methyl-2-naphthalene; naphthalene, 2-methyl; beta-methyl-naphthalene; DSSTox_CID_878; bmse000537; EC 202-078-3; DSSTox_RID_75842; DSSTox_GSID_20878; WLN: L66J C1; DTXSID4020878; NSC3575; 2-Methylnaphthalene (beta), 97%; AMY39000; ZINC1666853; Tox21_200839; AC-615; BDBM50159241; STL283950; AKOS000120035; ZINC112980287; CAS-91-57-6; NCGC00091415-01; NCGC00091415-02; NCGC00258393-01; 7419-61-6; BS-22305; DB-002463; FT-0613065; M0372; EN300-20114; 2-Methylnaphthalene 10 microg/mL in Cyclohexane; 2-Methylnaphthalene 10 microg/mL in Acetonitrile; Q2813819; W-100305; 2-Methylnaphthalene 1000 microg/mL in Dichloromethane; Z104476894
|
|
| CAS | 91-57-6 | |
| PubChem CID | 7055 | |
| ChEMBL ID | CHEMBL195895 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 142.2 | ALogp: | 3.9 |
| HBD: | 0 | HBA: | 0 |
| Rotatable Bonds: | 0 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 0.0 | Aromatic Rings: | 2 |
| Heavy Atoms: | 11 | QED Weighted: | 0.522 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.327 | MDCK Permeability: | 0.00001930 |
| Pgp-inhibitor: | 0.008 | Pgp-substrate: | 0.02 |
| Human Intestinal Absorption (HIA): | 0.003 | 20% Bioavailability (F20%): | 0.937 |
| 30% Bioavailability (F30%): | 0.399 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.761 | Plasma Protein Binding (PPB): | 95.83% |
| Volume Distribution (VD): | 1.091 | Fu: | 4.34% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.98 | CYP1A2-substrate: | 0.84 |
| CYP2C19-inhibitor: | 0.83 | CYP2C19-substrate: | 0.409 |
| CYP2C9-inhibitor: | 0.408 | CYP2C9-substrate: | 0.689 |
| CYP2D6-inhibitor: | 0.325 | CYP2D6-substrate: | 0.886 |
| CYP3A4-inhibitor: | 0.099 | CYP3A4-substrate: | 0.325 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 10.605 | Half-life (T1/2): | 0.343 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.073 | Human Hepatotoxicity (H-HT): | 0.083 |
| Drug-inuced Liver Injury (DILI): | 0.517 | AMES Toxicity: | 0.536 |
| Rat Oral Acute Toxicity: | 0.064 | Maximum Recommended Daily Dose: | 0.203 |
| Skin Sensitization: | 0.813 | Carcinogencity: | 0.821 |
| Eye Corrosion: | 0.906 | Eye Irritation: | 0.995 |
| Respiratory Toxicity: | 0.071 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000714 |  |
0.634 | D02NTO |  |
0.462 | ||
| ENC000392 |  |
0.571 | D02WCI | 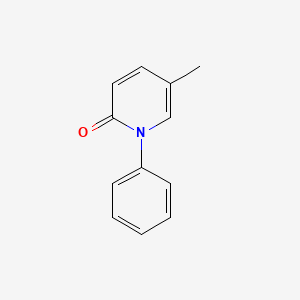 |
0.440 | ||
| ENC000167 | 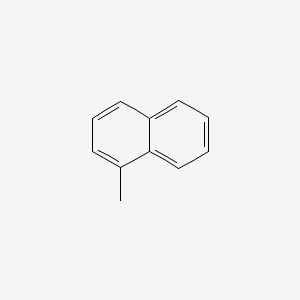 |
0.561 | D0J6WW | 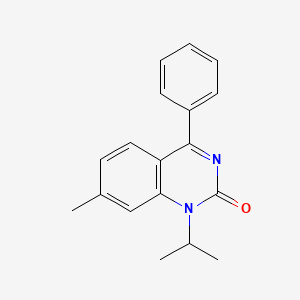 |
0.358 | ||
| ENC000047 | 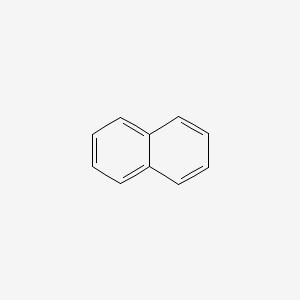 |
0.550 | D0O6IZ | 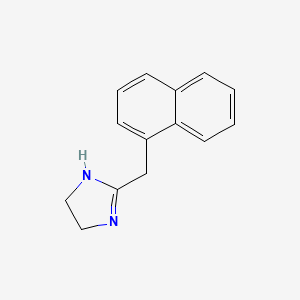 |
0.356 | ||
| ENC001367 | 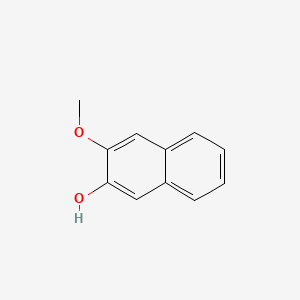 |
0.468 | D03WEX | 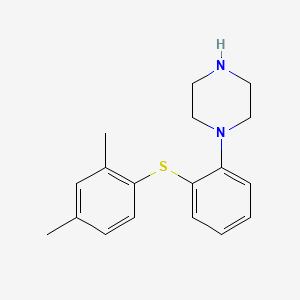 |
0.348 | ||
| ENC000025 | 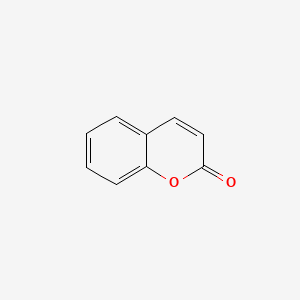 |
0.455 | D04JEE | 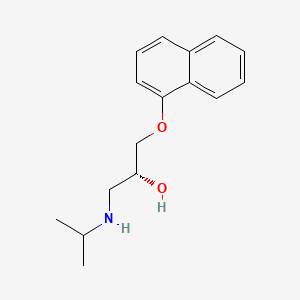 |
0.344 | ||
| ENC000178 | 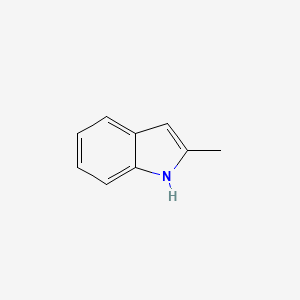 |
0.452 | D0BZ7W | 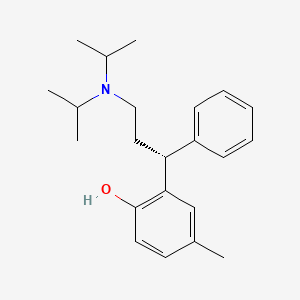 |
0.324 | ||
| ENC000892 | 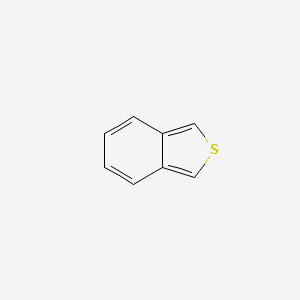 |
0.439 | D0A1PX | 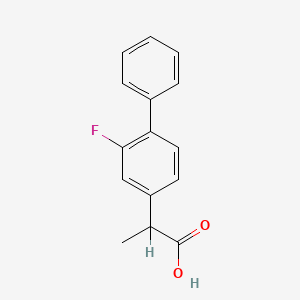 |
0.323 | ||
| ENC001388 | 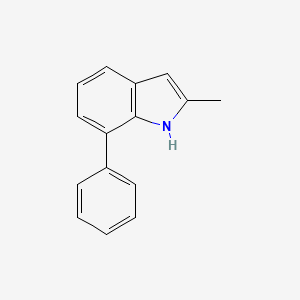 |
0.411 | D05FTJ | 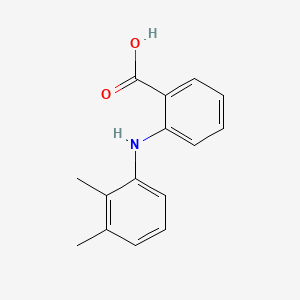 |
0.323 | ||
| ENC000064 | 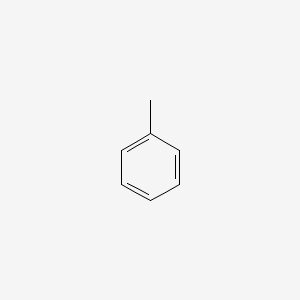 |
0.405 | D03XYW | 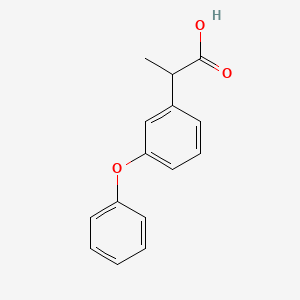 |
0.317 | ||