NPs Basic Information

|
Name |
Piperitone
|
| Molecular Formula | C10H16O | |
| IUPAC Name* |
3-methyl-6-propan-2-ylcyclohex-2-en-1-one
|
|
| SMILES |
CC1=CC(=O)C(CC1)C(C)C
|
|
| InChI |
InChI=1S/C10H16O/c1-7(2)9-5-4-8(3)6-10(9)11/h6-7,9H,4-5H2,1-3H3
|
|
| InChIKey |
YSTPAHQEHQSRJD-UHFFFAOYSA-N
|
|
| Synonyms |
PIPERITONE; 89-81-6; 3-Carvomenthenone; p-Menth-1-en-3-one; 6-Isopropyl-3-methylcyclohex-2-enone; 1-p-Menthen-3-one; 2-Cyclohexen-1-one, 3-methyl-6-(1-methylethyl)-; 1-Methyl-4-isopropyl-1-cyclohexen-3-one; 3-methyl-6-(propan-2-yl)cyclohex-2-en-1-one; DL-Piperitone; 3-methyl-6-propan-2-ylcyclohex-2-en-1-one; 3-methyl-6-(1-methylethyl)-2-cyclohexen-1-one; 1VZ8RG269R; CHEBI:48933; NSC-251528; 6-Isopropyl-3-methyl-2-cyclohexen-1-one; EINECS 201-942-7; NSC 251528; 2-Cyclohexen-1-one, 3-methyl-6-(1-methylethyl)-, (S)-; BRN 1907772; UNII-1VZ8RG269R; AI3-16053; (+-)-Piperitone; PIPERITONE [MI]; PIPERITONE, DL-; EC 201-942-7; 2-Cyclohexen-1-one, dimer; 2-07-00-00075 (Beilstein Handbook Reference); SCHEMBL111913; Piperitone, analytical standard; CHEMBL2252746; DTXSID7052604; FEMA 2910; 2-Cyclohexen-1-one,3-methyl-6-(1-methylethyl)-, (6S)-; NSC1100; FEMA NO. 2910, DL-; HY-N9496; NSC-1100; MFCD00045532; NSC176162; NSC251528; AKOS015840487; NSC-176162; AS-56754; DB-057169; 6-Isopropyl-3-methyl-2-cyclohexen-1-one #; CS-0181935; FT-0631428; FT-0697087; P2355; D92155; EN300-174688; Q2041498; 6-Isopropyl-3-methyl-2-cyclohexen-1-one predominantly
|
|
| CAS | 89-81-6 | |
| PubChem CID | 6987 | |
| ChEMBL ID | CHEMBL2252746 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 152.23 | ALogp: | 2.2 |
| HBD: | 0 | HBA: | 1 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 17.1 | Aromatic Rings: | 1 |
| Heavy Atoms: | 11 | QED Weighted: | 0.563 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.362 | MDCK Permeability: | 0.00002860 |
| Pgp-inhibitor: | 0.005 | Pgp-substrate: | 0.002 |
| Human Intestinal Absorption (HIA): | 0.004 | 20% Bioavailability (F20%): | 0.01 |
| 30% Bioavailability (F30%): | 0.151 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.906 | Plasma Protein Binding (PPB): | 89.34% |
| Volume Distribution (VD): | 1.031 | Fu: | 10.14% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.359 | CYP1A2-substrate: | 0.789 |
| CYP2C19-inhibitor: | 0.351 | CYP2C19-substrate: | 0.93 |
| CYP2C9-inhibitor: | 0.212 | CYP2C9-substrate: | 0.839 |
| CYP2D6-inhibitor: | 0.022 | CYP2D6-substrate: | 0.673 |
| CYP3A4-inhibitor: | 0.071 | CYP3A4-substrate: | 0.603 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 5.322 | Half-life (T1/2): | 0.693 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.013 | Human Hepatotoxicity (H-HT): | 0.158 |
| Drug-inuced Liver Injury (DILI): | 0.082 | AMES Toxicity: | 0.032 |
| Rat Oral Acute Toxicity: | 0.043 | Maximum Recommended Daily Dose: | 0.081 |
| Skin Sensitization: | 0.788 | Carcinogencity: | 0.612 |
| Eye Corrosion: | 0.888 | Eye Irritation: | 0.981 |
| Respiratory Toxicity: | 0.786 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC001823 | 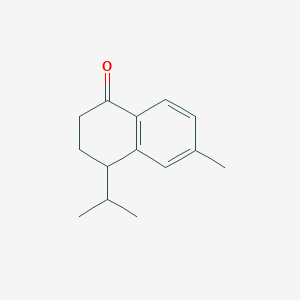 |
0.429 | D04CSZ | 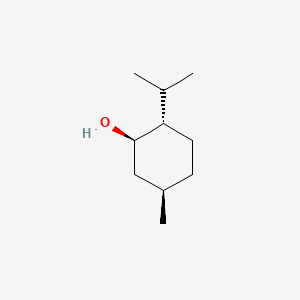 |
0.234 | ||
| ENC000762 |  |
0.415 | D0H1QY |  |
0.208 | ||
| ENC000763 |  |
0.415 | D06GIP | 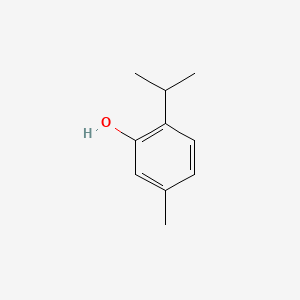 |
0.208 | ||
| ENC001837 |  |
0.381 | D0TY5N |  |
0.200 | ||
| ENC001817 | 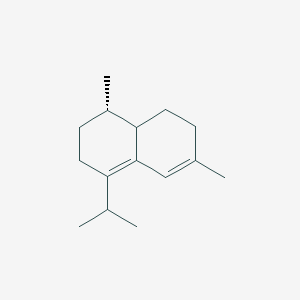 |
0.373 | D0K7LU | 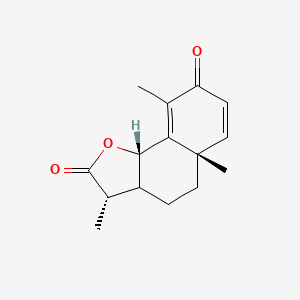 |
0.200 | ||
| ENC000802 |  |
0.356 | D0P4MT |  |
0.200 | ||
| ENC001824 | 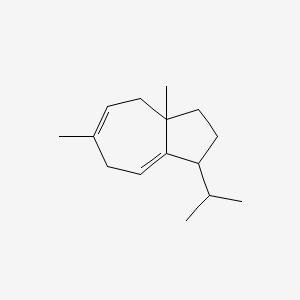 |
0.346 | D04ATM | 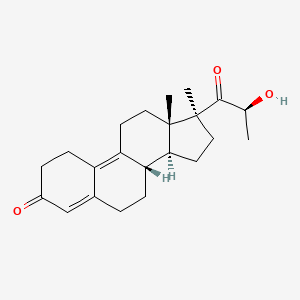 |
0.195 | ||
| ENC003090 |  |
0.346 | D09PJX | 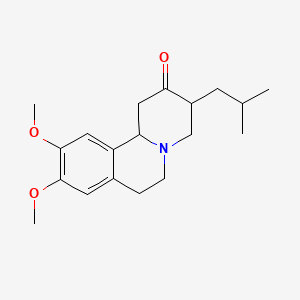 |
0.192 | ||
| ENC002227 |  |
0.346 | D0Z8SF | 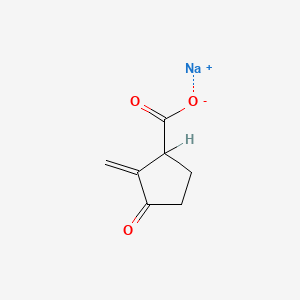 |
0.191 | ||
| ENC002224 |  |
0.346 | D06IXT | 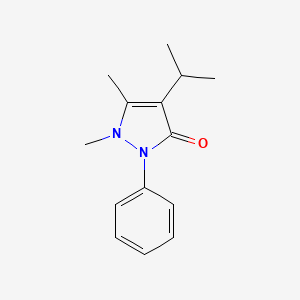 |
0.190 | ||