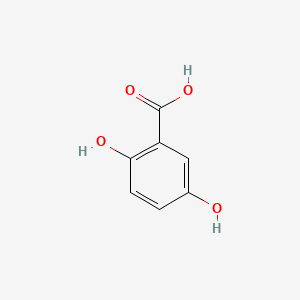
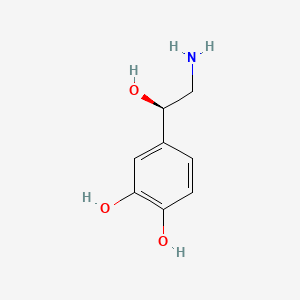
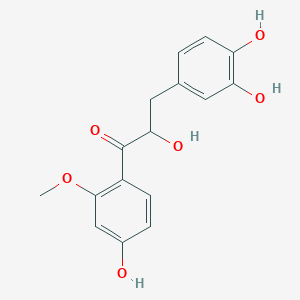
| Synonyms |
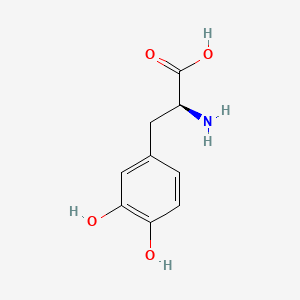
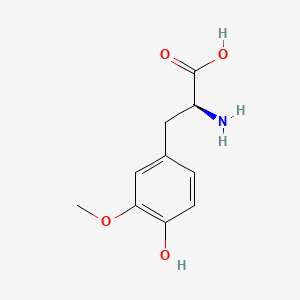
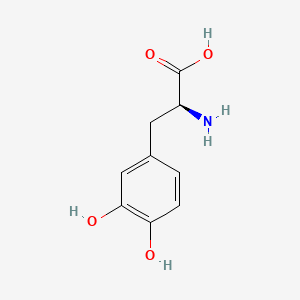
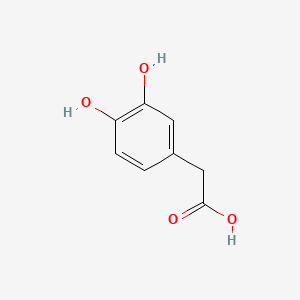
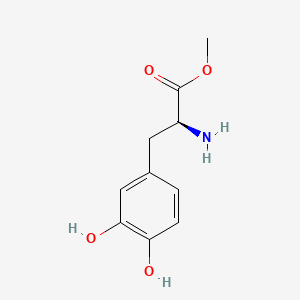
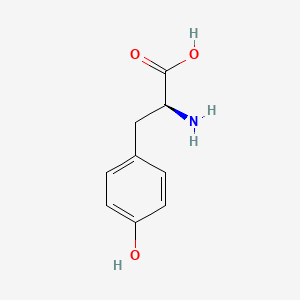
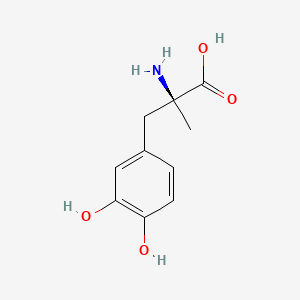
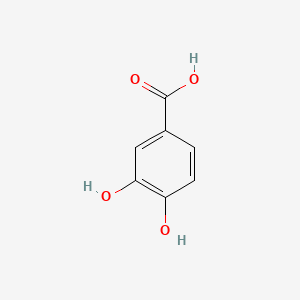
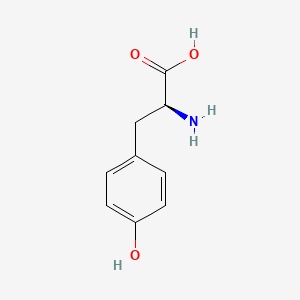
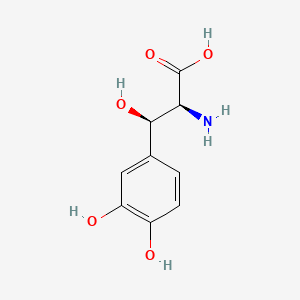
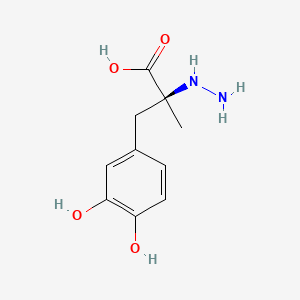
levodopa; L-dopa; 59-92-7; Dopar; 3,4-dihydroxy-L-phenylalanine; 3-Hydroxy-L-tyrosine; Bendopa; Larodopa; Levopa; 3,4-Dihydroxyphenylalanine; Brocadopa; Cidandopa; Insulamina; Maipedopa; Dopaidan; Dopalina; Dopasol; Eldopal; Eldopar; Pardopa; Prodopa; Syndopa; 3-(3,4-Dihydroxyphenyl)-L-alanine; (-)-Dopa; Dihydroxy-L-phenylalanine; Helfo-Dopa; Dopaflex; Deadopa; Dopal-fher; Doparkine; Dopaston; Dopastral; Eldopatec; Eurodopa; Doparl; Doprin; Veldopa; L-3,4-Dihydroxyphenylalanine; (2S)-2-amino-3-(3,4-dihydroxyphenyl)propanoic acid; Levedopa; Levodopum; L-o-Hydroxytyrosine; L-Tyrosine, 3-hydroxy-; Dopa; (-)-3-(3,4-Dihydroxyphenyl)-L-alanine; Ledopa; 3,4-Dihydroxyphenyl-L-alanine; Dopaston SE; beta-(3,4-Dihydroxyphenyl)-L-alanine; L-(o-Dihydroxyphenyl)alanine; L-(-)-Dopa; L-3-Hydroxytyrosine; L-beta-(3,4-Dihydroxyphenyl)alanine; Weldopa; Parda; L-Dihydroxyphenylalanine; L-3-(3,4-Dihydroxyphenyl)alanine; (S)-2-amino-3-(3,4-dihydroxyphenyl)propanoic acid; Ro 4-6316; beta-(3,4-Dihydroxyphenyl)alanine; Inbrija; CVT-301; alanine, 3-(3,4-dihydroxyphenyl)-, L-; dihydroxyphenylalanine; component of Sinemet; Dopar (TN); CHEBI:15765; beta-(3,4-Dihydroxyphenyl)-alpha-L-alanine; L-beta-(3,4-Dihydroxyphenyl)-alpha-alanine; Alanine, 3-(3,4-dihydroxyphenyl)-, (-)-; L(-)-Dopa; (-)-(3,4-Dihydroxyphenyl)alanine; NSC-118381; L-.beta.-(3,4-Dihydroxyphenyl)alanine; CHEMBL1009; .beta.-(3,4-Dihydroxyphenyl)-L-alanine; L-(3,4-Dihydroxyphenyl)alanine; NSC118381; .beta.-(3,4-Dihydroxyphenyl)alanine; CAS-59-92-7; Tyrosine, 3-hydroxy-; NCGC00016270-04; Biodopa; Cerepap; Laradopa; Sobiodopa; L-(3,4-Dihydroxyphenyl)-.alpha.-alanine; 46627O600J; MFCD00002598; V-1512; C9H11NO4; Helfo DOPA; 65170-01-6; beta-(3,4-Dihydroxyphenyl)-alpha-alanine; Atamet; Levodopum [INN-Latin]; BDBM50130192; L-O-Dihydroxyphenylalanine; L Dopa; L-3; CCRIS 3766; HSDB 3348; WLN: QVYZ1R CQ DQ; 3,4-Dihydroxyphenylalanine (VAN); SR-01000075384; EINECS 200-445-2; NSC 118381; L-3,4-Dihydrophenylalanine; Dopastone; Dopicar; Prolopa; (2S)-2-amino-3-(3,4-dihydroxyphenyl)propanoate; UNII-46627O600J; Prestwick_185; Levodopa (Sinemet); L-DOPA; Levodopa; Madopa (Salt/Mix); Levodopa [USAN:USP:INN:BAN:JAN]; Spectrum_000454; 4-dihydroxyphenylalanine; LEVODOPA [HSDB]; LEVODOPA [USAN]; LEVODOPA [INN]; LEVODOPA [JAN]; Carbidopa EP Impurity A; LEVODOPA [MI]; LEVODOPA [VANDF]; Prestwick0_000017; Prestwick1_000017; Prestwick2_000017; Prestwick3_000017; Spectrum2_000496; Spectrum4_000539; Spectrum5_001899; Lopac-D-9628; Levodopa (JP15/USP); DSSTox_CID_3209; LEVODOPA [MART.]; bmse000322; Epitope ID:150927; LEVODOPA [USP-RS]; LEVODOPA [WHO-DD]; LEVODOPA [WHO-IP]; DOPA, L-; 3, 4-Dihydroxyphenylalanine; Alanine,4-dihydroxyphenyl)-; DSSTox_RID_76926; LEVODOPA [EMA EPAR]; DSSTox_GSID_23209; Lopac0_000454; SCHEMBL22655; BSPBio_000053; BSPBio_002354; KBioGR_001177; KBioSS_000934; L-4-5-Dihydroxyphenylalanine; MLS000028514; BIDD:GT0158; DivK1c_000452; SPECTRUM2300205; Levodopa (JP17/USP/INN); SPBio_000391; SPBio_001974; DHIVY COMPONENT LEVODOPA; DUOPA COMPONENT LEVODOPA; LEVODOPA [EP IMPURITY]; LEVODOPA [ORANGE BOOK]; BPBio1_000059; GTPL3639; LEVODOPA [EP MONOGRAPH]; RYTARY COMPONENT LEVODOPA; b-(3,4-Dihydroxyphenyl)alanine; DTXSID9023209; LEVODOPA [USP MONOGRAPH]; WLN: QVYZ1R CQ DQ -L; 3, 4-Dihydroxy-L-phenylalanine; BDBM60928; HMS501G14; KBio1_000452; KBio2_000934; KBio2_003502; KBio2_006070; PARCOPA COMPONENT LEVODOPA; STALEVO COMPONENT LEVODOPA; LEVODOPUM [WHO-IP LATIN]; Alanine,4-dihydroxyphenyl)-, L-; CARBILEV COMPONENT LEVODOPA; CORBILTA COMPONENT LEVODOPA; DOPASNAP COMPONENT LEVODOPA; IPX203 COMPONENT LEVODOPA; L-(3, 4-Dihydroxyphenyl)alanine; NINDS_000452; HMS1568C15; HMS1922J14; HMS2090O08; HMS2093N04; HMS2095C15; HMS2230B04; HMS3261K10; HMS3712C15; LEVODOPA COMPONENT OF DHIVY; LEVODOPA COMPONENT OF DUOPA; Pharmakon1600-02300205; ZINC895199; H-Phe{3,4-(OH)2}-OH; HY-N0304; IPX-203 COMPONENT LEVODOPA; LEVODOPA COMPONENT OF RYTARY; Levodopa;3,4-Dihydroxyphenylalanine; b-(3,4-Dihydroxyphenyl)-L-alanine; Inbrija (levodopa inhalation powder); LEVODOPA COMPONENT OF PARCOPA; LEVODOPA COMPONENT OF SINEMET; LEVODOPA COMPONENT OF STALEVO; Tox21_110338; Tox21_500454; CCG-39571; L-3-(3,4-dihydroxy-phenyl)alanine; L-3-(3,4-dihydroxyphenyl)-Alanine; NSC759573; PDSP1_001541; PDSP2_001525; s1726; Alanine, 3-(3,4-dihydroxyphenyl)-; Alanine,4-dihydroxyphenyl)-, (-)-; LEVODOPA COMPONENT OF CARBILEV; LEVODOPA COMPONENT OF CORBILTA; LEVODOPA COMPONENT OF DOPASNAP; AKOS010396267; b-(3,4-Dihydroxyphenyl)-a-L-alanine; L-b-(3,4-Dihydroxyphenyl)-a-alanine; .beta.-(3, 4-Dihydroxyphenyl)alanine; AC-8432; AM82124; CS-1945; DB01235; LP00454; NSC-759573; SDCCGMLS-0066924.P001; SDCCGSBI-0050439.P004; IDI1_000452; NCGC00015384-01; NCGC00016270-01; NCGC00016270-06; NCGC00016270-07; NCGC00016270-09; NCGC00016270-10; NCGC00016270-22; NCGC00093869-04; NCGC00261139-01; AS-13287; BP-12850; SMR000058312; SBI-0050439.P003; L-(3, 4-Dihydroxyphenyl)-.alpha.-alanine; D0600; D9628; EU-0100454; EN300-52637; 59L927; Alanine, 3-(3, 4-dihydroxyphenyl)-, (-)-; C00355; D 9628; D00059; D70595; 3,4-Dihydroxy-L-phenylalanine, >=98% (TLC); AB00052418-06; AB00052418-07; AB00052418_08; AB00052418_09; A832543; Q300989; (2S)-2-amino-3-(3,4-dihydroxyphenyl)propanoicacid; Q-201294; SR-01000075384-1; SR-01000075384-4; SR-01000075384-6; SR-01000075384-7; (S)-2-Amino-3-(3,4-dihydroxy-phenyl)-propionic acid; F0347-4695; Levodopa, British Pharmacopoeia (BP) Reference Standard; Levodopa, European Pharmacopoeia (EP) Reference Standard; Z756440064; (2S)-2-amino-3-(3,4-dihydroxyphenyl)propanoic acidL-dopa; 1E83F927-C221-46AA-B90A-81B33C5F3868; 3,4-Dihydroxy-L-phenylalanine, Vetec(TM) reagent grade, 98%; LEVODOPA COMPONENT OF LEVODOPA/CARBIDOPA/ENTACAPONE ORION; Levodopa, United States Pharmacopeia (USP) Reference Standard; LEVODOPA/CARBIDOPA/ENTACAPONE ORION COMPONENT LEVODOPA; 3,4-Dihydroxy-L-phenylalanine, certified reference material, TraceCERT(R); Levodopa, Pharmaceutical Secondary Standard; Certified Reference Material; 122769-74-8; L-Methyldopa ; (2S)-2-Amino-3-(3,4-dihydroxyphenyl)-2-methylpropanoic acid; 3-(3,4-Dihydroxyphenyl)-?-methyl-L-alanine; 3-Hydroxy-a-methyl-L-tyrosine
|