NPs Basic Information
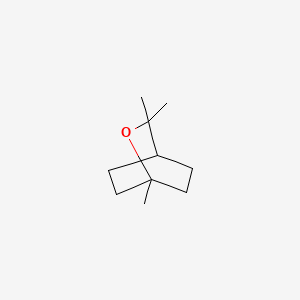
|
Name |
Eucalyptol
|
| Molecular Formula | C10H18O | |
| IUPAC Name* |
1,3,3-trimethyl-2-oxabicyclo[2.2.2]octane
|
|
| SMILES |
CC1(C2CCC(O1)(CC2)C)C
|
|
| InChI |
InChI=1S/C10H18O/c1-9(2)8-4-6-10(3,11-9)7-5-8/h8H,4-7H2,1-3H3
|
|
| InChIKey |
WEEGYLXZBRQIMU-UHFFFAOYSA-N
|
|
| Synonyms |
Eucalyptol; cineole; 1,8-Cineole; 470-82-6; 1,8-Cineol; Cajeputol; 1,8-Epoxy-p-menthane; Eucalyptole; Eucapur; Zineol; Terpan; p-Cineole; 1,3,3-Trimethyl-2-oxabicyclo[2.2.2]octane; Eukalyptol; 1,8-Oxido-p-menthane; CINEOL; Cucalyptol; Soledum; p-Menthane, 1,8-epoxy-; Eukalyptol [Czech]; Eucalyptol (natural); FEMA No. 2465; 2-Oxabicyclo[2.2.2]octane, 1,3,3-trimethyl-; 8000-48-4; Cineole (VAN); NCI-C56575; 2-Oxabicyclo(2.2.2)octane, 1,3,3-trimethyl-; Eucaly; 1,3,3-Trimethyl-2-oxabicyclo(2.2.2)octane; 2-Oxa-1,3,3-trimethylbicyclo(2.2.2)octane; NSC 6171; NSC-6171; NSC6171; 2,2,4-trimethyl-3-oxabicyclo[2.2.2]octane; 2-Oxa-1,3,3-trimethylbicyclo[2.2.2]octane; RV6J6604TK; CNL; 4,7,7-trimethyl-8-oxabicyclo[2.2.2]octane; CHEBI:27961; Eucalyptol [USAN]; NCGC00091666-01; NCGC00091666-04; DSSTox_CID_616; DSSTox_RID_75692; DSSTox_GSID_20616; (1s,4s)-1,3,3-trimethyl-2-oxabicyclo[2.2.2]octane; Eucalyptol 1000 microg/mL in Methanol; UNII-RV6J6604TK; CAS-470-82-6; SMR000471853; CCRIS 3727; HSDB 991; Eucalyptol [USAN:USP]; EINECS 207-431-5; MFCD00167977; Terpane; Cyneol; BIDD:ER0481; AI3-00578; Eucalyptol,(S); Eucalyptol (USP); 1.8-cineole; Eucalyptol, 99%; Eucalyptol, Ph Helv; p-Menthane,8-epoxy-; cineole (1,8-); EUCALYPTOL [II]; EUCALYPTOL [MI]; WLN: T66 A B AOTJ B1 B1 F1; CINEOLE [INCI]; EUCALYPTOL [FCC]; 1,8-Cineol-[d3]; CINEOLE [MART.]; Spectrum2_000221; Spectrum3_000683; Spectrum4_001747; Spectrum5_000704; EUCALYPTOL [FHFI]; EUCALYPTOL [HPUS]; EUCALYPTOL [HSDB]; EUCALYPTOL [INCI]; CINEOLE [WHO-DD]; EUCALYPTOL [VANDF]; bmse000523; EC 207-431-5; EUCALYPTOL [USP-RS]; SCHEMBL19622; SCHEMBL41020; BSPBio_002405; KBioGR_002194; MLS001050089; MLS001066338; DivK1c_000333; SPECTRUM1500294; SPBio_000261; CINEOLE [EP MONOGRAPH]; Eucalyptol, analytical standard; CHEMBL485259; GTPL2464; CHEMBL1231862; CHEMBL1397305; DTXSID4020616; SCHEMBL13554591; SCHEMBL17836873; HMS501A15; KBio1_000333; KBio3_001625; EUCALYPTOL [USP IMPURITY]; NINDS_000333; EUCALYPTOL [USP MONOGRAPH]; HMS2271P04; Pharmakon1600-01500294; ZINC967566; HY-N0066; Tox21_111161; Tox21_202090; Tox21_302902; BDBM50459887; CCG-36080; NSC760388; AKOS015903223; AKOS016034339; AKOS037514637; Tox21_111161_1; CCG-266254; CS-8146; DB03852; LMPR0102090019; NSC-760388; IDI1_000333; Eucalyptol, tested according to Ph.Eur.; NCGC00091666-02; NCGC00091666-03; NCGC00091666-05; NCGC00095774-01; NCGC00178671-01; NCGC00256479-01; NCGC00259639-01; AC-20234; Eucalyptol, natural, >=99%, FCC, FG; LS-13868; NCI60_005108; 1,3-Trimethyl-2-oxabicyclo[2.2.2]octane; 2-Oxa-1,3-trimethylbicyclo[2.2.2]octane; DB-070775; 2-Oxabicyclo[2.2.2]octane,3,3-trimethyl-; FT-0607033; FT-0626369; 1,3,3-trimethyl-2-oxabicyclo[2,2,2]octane; A15662; C09844; D04115; AB01563262_01; Q161572; SR-01000763816; SR-01000763816-2; W-106080; 1,8-Cineole, primary pharmaceutical reference standard; Cineole, European Pharmacopoeia (EP) Reference Standard; Eucalyptol, certified reference material, TraceCERT(R); F0001-1260; Eucalyptol, United States Pharmacopeia (USP) Reference Standard; Eucalyptol (cineole), Pharmaceutical Secondary Standard; Certified Reference Material
|
|
| CAS | 470-82-6 | |
| PubChem CID | 2758 | |
| ChEMBL ID | CHEMBL485259 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 154.25 | ALogp: | 2.5 |
| HBD: | 0 | HBA: | 1 |
| Rotatable Bonds: | 0 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 9.2 | Aromatic Rings: | 3 |
| Heavy Atoms: | 11 | QED Weighted: | 0.518 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.414 | MDCK Permeability: | 0.00001910 |
| Pgp-inhibitor: | 0.002 | Pgp-substrate: | 0 |
| Human Intestinal Absorption (HIA): | 0.002 | 20% Bioavailability (F20%): | 0.01 |
| 30% Bioavailability (F30%): | 0.006 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.853 | Plasma Protein Binding (PPB): | 90.09% |
| Volume Distribution (VD): | 2.434 | Fu: | 16.87% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.095 | CYP1A2-substrate: | 0.569 |
| CYP2C19-inhibitor: | 0.235 | CYP2C19-substrate: | 0.914 |
| CYP2C9-inhibitor: | 0.136 | CYP2C9-substrate: | 0.804 |
| CYP2D6-inhibitor: | 0.016 | CYP2D6-substrate: | 0.549 |
| CYP3A4-inhibitor: | 0.009 | CYP3A4-substrate: | 0.234 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 8.066 | Half-life (T1/2): | 0.352 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.026 | Human Hepatotoxicity (H-HT): | 0.448 |
| Drug-inuced Liver Injury (DILI): | 0.046 | AMES Toxicity: | 0.01 |
| Rat Oral Acute Toxicity: | 0.045 | Maximum Recommended Daily Dose: | 0.074 |
| Skin Sensitization: | 0.199 | Carcinogencity: | 0.7 |
| Eye Corrosion: | 0.722 | Eye Irritation: | 0.948 |
| Respiratory Toxicity: | 0.182 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC005519 | 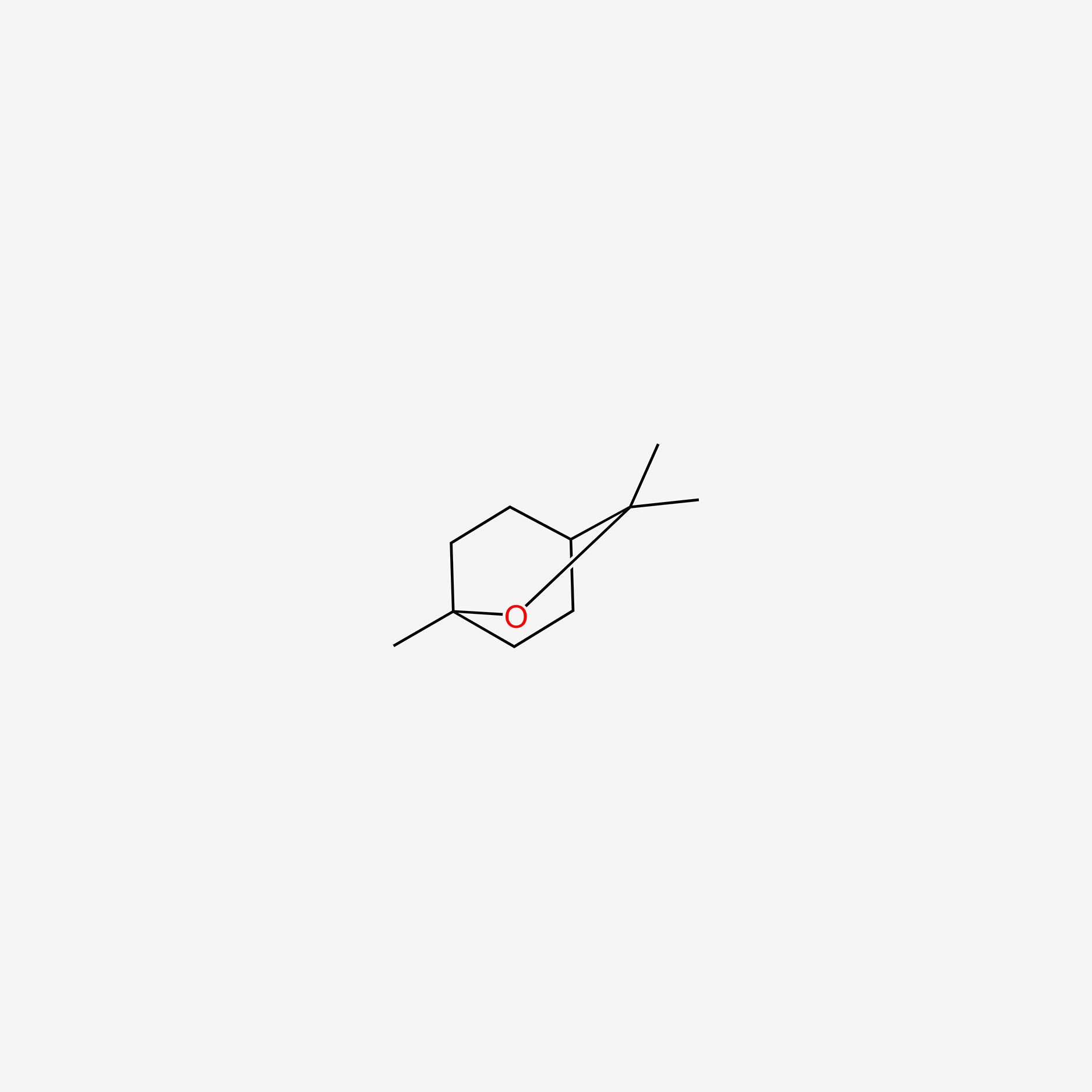 |
1.000 | D0H1QY |  |
0.372 | ||
| ENC001810 | 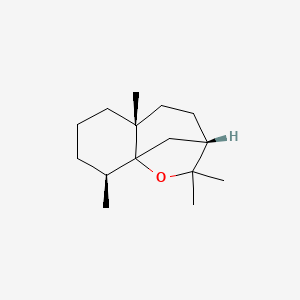 |
0.451 | D0V8HA | 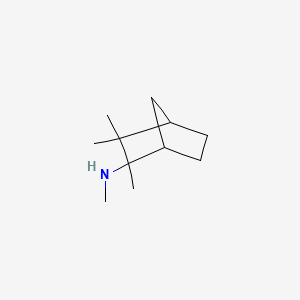 |
0.319 | ||
| ENC003049 | 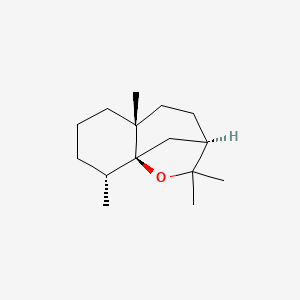 |
0.451 | D07QKN | 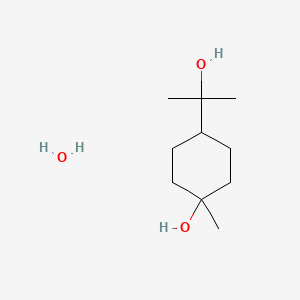 |
0.292 | ||
| ENC002074 | 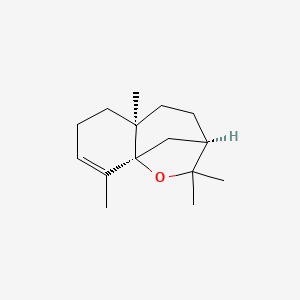 |
0.423 | D0U3GL | 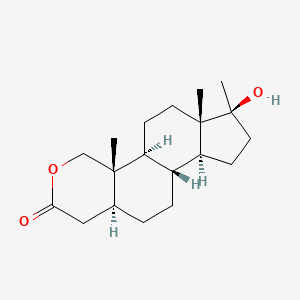 |
0.264 | ||
| ENC002337 |  |
0.423 | D0Q6NZ |  |
0.231 | ||
| ENC000481 |  |
0.372 | D0Z1XD |  |
0.230 | ||
| ENC001814 | 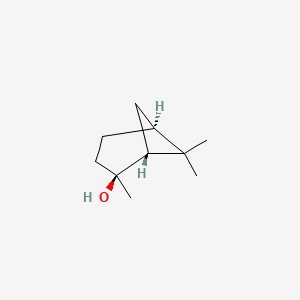 |
0.372 | D08QKJ | 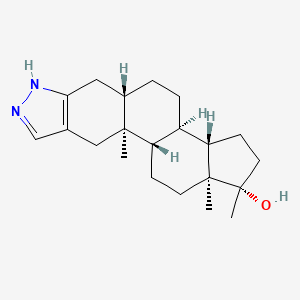 |
0.225 | ||
| ENC000331 | 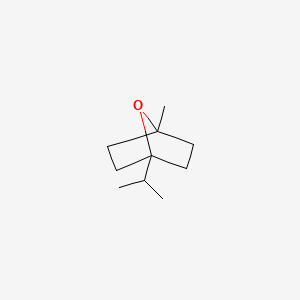 |
0.364 | D00VZZ | 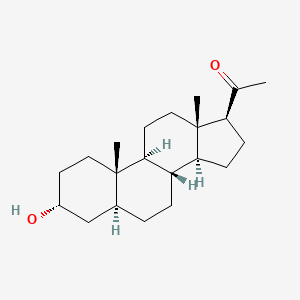 |
0.221 | ||
| ENC001196 | 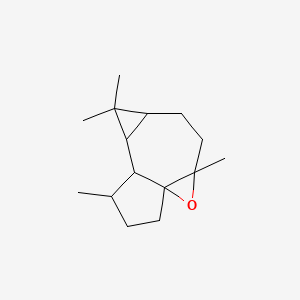 |
0.345 | D04DJN | 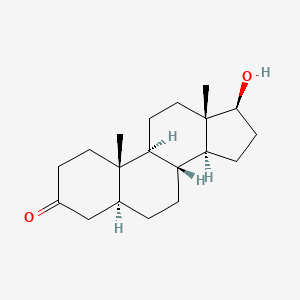 |
0.219 | ||
| ENC002112 | 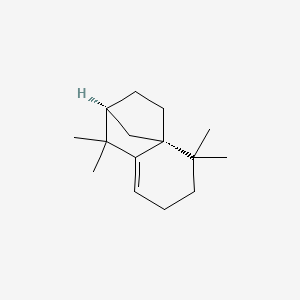 |
0.340 | D0L2LS | 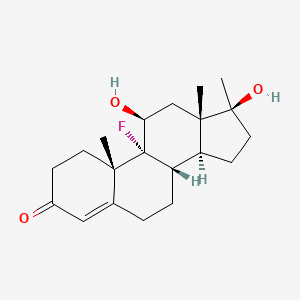 |
0.218 | ||