NPs Basic Information

|
Name |
Azelaic Acid
|
| Molecular Formula | C9H16O4 | |
| IUPAC Name* |
nonanedioic acid
|
|
| SMILES |
C(CCCC(=O)O)CCCC(=O)O
|
|
| InChI |
InChI=1S/C9H16O4/c10-8(11)6-4-2-1-3-5-7-9(12)13/h1-7H2,(H,10,11)(H,12,13)
|
|
| InChIKey |
BDJRBEYXGGNYIS-UHFFFAOYSA-N
|
|
| Synonyms |
azelaic acid; NONANEDIOIC ACID; 123-99-9; Finacea; Anchoic acid; Azelex; Lepargylic acid; 1,7-Heptanedicarboxylic acid; Skinoren; 1,9-Nonanedioic acid; Heptanedicarboxylic acid; n-Nonanedioic acid; Emerox 1110; Emerox 1144; azelate; acide azelaique; Finevin; Azelainic acid; acidum azelaicum; Skinorem; 1,7-Dicarboxyheptane; Azelaic acid, technical grade; Emery's L-110; ZK 62498; ZK-62498; 26776-28-3; NSC 19493; CHEBI:48131; NSC-19493; Water-soluble azelaic acid; F2VW3D43YT; MLS000069659; azelaate; NSC19493; MFCD00004432; NCGC00014993-07; SMR000059164; Acido azelaico; Azalaic Acid; DSSTox_CID_1640; Acide azelaique [French]; Acido azelaico [Spanish]; Acidum azelaicum [Latin]; DSSTox_RID_76254; DSSTox_GSID_21640; heptane-1,7-dicarboxylic acid; Azelaic acid [USAN:INN]; Azelaic; CAS-123-99-9; Finacea (TN); Azelex (TN); SR-01000075671; EINECS 204-669-1; UNII-F2VW3D43YT; Azelaic acid (USAN/INN); BRN 1101094; Azelaicacidtech; Azelainsaeure; Lepargylate; Nonandisaeure; Anchoate; azelaic-acid; n-Nonanedioate; AI3-06299; HSDB 7659; 1tuf; 1,9-Nonanedioate; SH-441; AGN-191861; Azelaic acid, 98%; Spectrum_000057; Water-solubleazelaicacid; Opera_ID_740; 1,7-Heptanedicarboxylate; Spectrum2_000995; Spectrum3_000278; Spectrum4_000401; Spectrum5_001304; AZELAIC ACID [MI]; Epitope ID:187039; A-9800; EC 204-669-1; AZELAIC ACID [INN]; Lopac-246379; SCHEMBL3887; AZELAIC ACID [HSDB]; AZELAIC ACID [INCI]; AZELAIC ACID [USAN]; CHEMBL1238; Lopac0_000051; AZELAIC ACID [VANDF]; BSPBio_001756; KBioGR_000662; KBioSS_000437; Nonanedioic acid Azelaic acid; 4-02-00-02055 (Beilstein Handbook Reference); MLS001148615; AZELAIC ACID [MART.]; BIDD:GT0315; DivK1c_000532; SPECTRUM1500648; SPBio_001089; AZELAIC ACID [WHO-DD]; GTPL7484; DTXSID8021640; HMS501K14; KBio1_000532; KBio2_000437; KBio2_003005; KBio2_005573; KBio3_001256; Azelaic acid, analytical standard; NINDS_000532; HMS1921O11; HMS2092E22; HMS2234D10; HMS3260K03; HMS3372J07; Pharmakon1600-01500648; AZELAIC ACID [ORANGE BOOK]; BCP18690; HY-B0704; ZINC1531036; Tox21_110063; Tox21_201989; Tox21_303011; Tox21_500051; Azelaic acid, technical grade, 80%; CCG-40081; LMFA01170054; NSC757406; s4550; STL059432; AKOS000120052; Tox21_110063_1; Azelaic acid, technical, ~85% (GC); Azelaic acid, Vetec(TM) reagent grade; DB00548; KS-5293; LP00051; NSC-757406; SDCCGMLS-0066619.P001; SDCCGMLS-0066619.P033; SDCCGSBI-0050040.P004; IDI1_000532; MLS-0066619; NCGC00014993-01; NCGC00014993-02; NCGC00014993-03; NCGC00014993-04; NCGC00014993-05; NCGC00014993-06; NCGC00014993-08; NCGC00014993-09; NCGC00014993-10; NCGC00014993-12; NCGC00014993-15; NCGC00093565-01; NCGC00093565-02; NCGC00093565-03; NCGC00093565-04; NCGC00093565-05; NCGC00093565-06; NCGC00093565-07; NCGC00256508-01; NCGC00259538-01; NCGC00260736-01; BP-27863; MLS-0066619.P021; SBI-0050040.P003; A0561; Dicarboxylic acid C9; Nonanedioic acid; AZA; EU-0100051; FT-0626920; EN300-18040; C08261; D03034; D70171; AB00052140_12; Q413504; SR-01000075671-1; SR-01000075671-4; SR-01000075671-6; 0C50D8EC-0DB0-4F24-8EFC-2919E1F0D9BF; Z57127532; F8889-5093
|
|
| CAS | 123-99-9 | |
| PubChem CID | 2266 | |
| ChEMBL ID | CHEMBL1238 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 188.22 | ALogp: | 1.6 |
| HBD: | 2 | HBA: | 4 |
| Rotatable Bonds: | 8 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 74.6 | Aromatic Rings: | 0 |
| Heavy Atoms: | 13 | QED Weighted: | 0.574 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -5.745 | MDCK Permeability: | 0.00008720 |
| Pgp-inhibitor: | 0.001 | Pgp-substrate: | 0.001 |
| Human Intestinal Absorption (HIA): | 0.068 | 20% Bioavailability (F20%): | 0.056 |
| 30% Bioavailability (F30%): | 0.98 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.043 | Plasma Protein Binding (PPB): | 83.89% |
| Volume Distribution (VD): | 0.26 | Fu: | 15.20% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.017 | CYP1A2-substrate: | 0.073 |
| CYP2C19-inhibitor: | 0.023 | CYP2C19-substrate: | 0.052 |
| CYP2C9-inhibitor: | 0.009 | CYP2C9-substrate: | 0.972 |
| CYP2D6-inhibitor: | 0.014 | CYP2D6-substrate: | 0.106 |
| CYP3A4-inhibitor: | 0.006 | CYP3A4-substrate: | 0.006 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 1.727 | Half-life (T1/2): | 0.85 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.005 | Human Hepatotoxicity (H-HT): | 0.073 |
| Drug-inuced Liver Injury (DILI): | 0.048 | AMES Toxicity: | 0.008 |
| Rat Oral Acute Toxicity: | 0.008 | Maximum Recommended Daily Dose: | 0.018 |
| Skin Sensitization: | 0.13 | Carcinogencity: | 0.137 |
| Eye Corrosion: | 0.987 | Eye Irritation: | 0.983 |
| Respiratory Toxicity: | 0.04 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC001325 |  |
0.688 | D0E4WR | 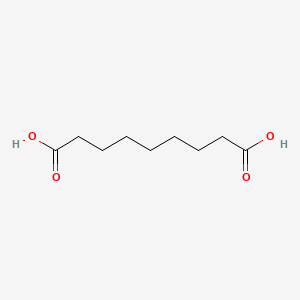 |
1.000 | ||
| ENC000263 | 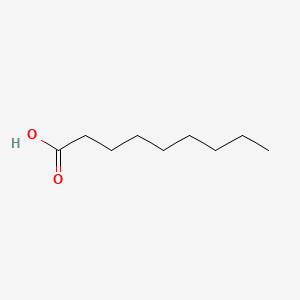 |
0.625 | D0Z5BC |  |
0.543 | ||
| ENC000647 | 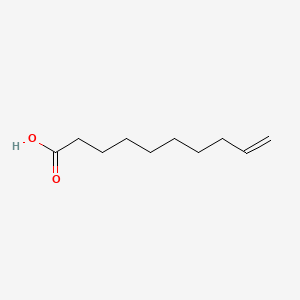 |
0.581 | D06VNK | 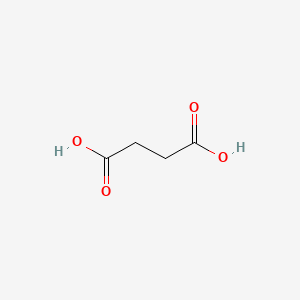 |
0.486 | ||
| ENC000088 | 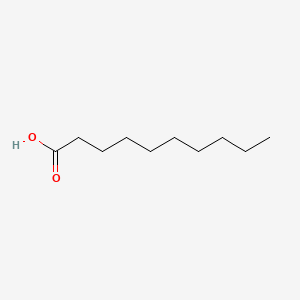 |
0.581 | D0FD0H | 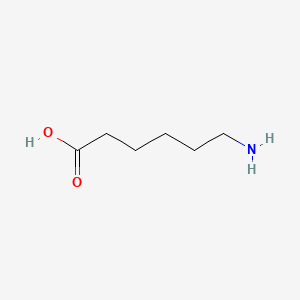 |
0.475 | ||
| ENC000691 | 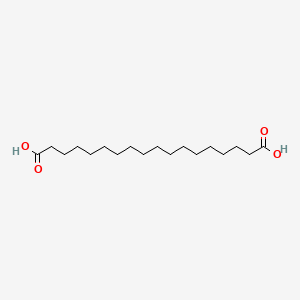 |
0.565 | D0XN8C |  |
0.382 | ||
| ENC001913 | 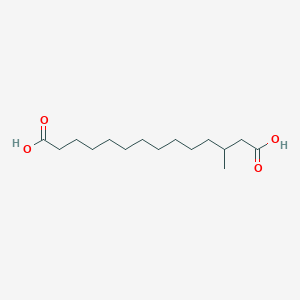 |
0.554 | D0EP8X | 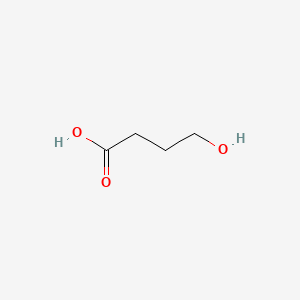 |
0.359 | ||
| ENC000030 | 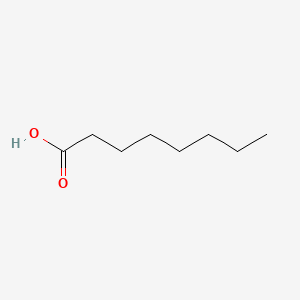 |
0.550 | D09SRR | 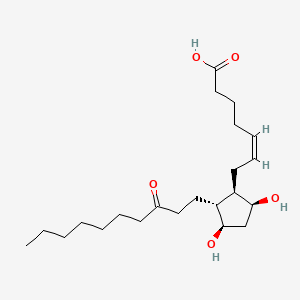 |
0.358 | ||
| ENC000270 | 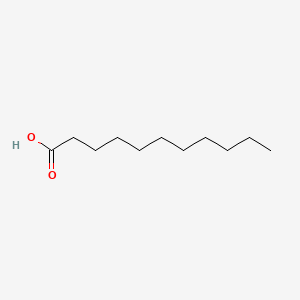 |
0.543 | D0Y7ZD | 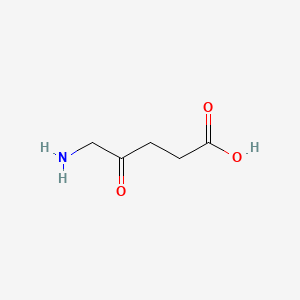 |
0.349 | ||
| ENC000593 | 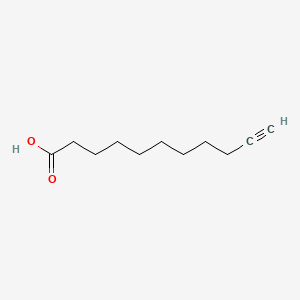 |
0.543 | D0E7PQ | 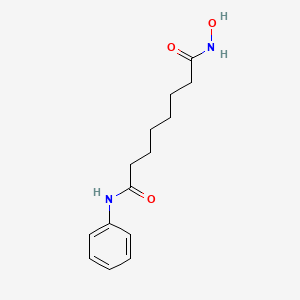 |
0.348 | ||
| ENC000516 | 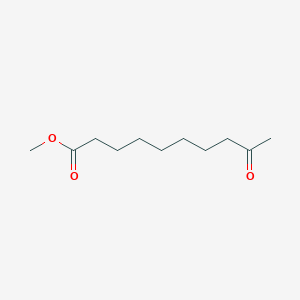 |
0.521 | D0O1PH |  |
0.347 | ||