| InChI |
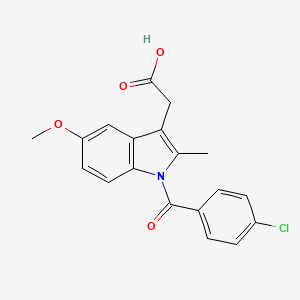
InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23)
|
| Synonyms |
indomethacin; 53-86-1; indometacin; Indocin; Indomethacine; Indometacine; Indocid; Metindol; Reumacide; Indomethazine; Imbrilon; Amuno; Tannex; Indomethacinum; Artracin; Artrinovo; Artrivia; Confortid; Idomethine; Indomecol; Indoptic; Indoptol; Inflazon; Infrocin; Metartril; Methazine; Mikametan; Sadoreum; Dolovin; Inacid; Indacin; Indomed; Indomee; Lausit; Metacen; Mobilan; Indo-rectolmin; Indo-tablinen; Inteban sp; Durametacin; Indometacyna; Indometicina; Mezolin; Indo-Lemmon; Indometacinum; 2-(1-(4-Chlorobenzoyl)-5-methoxy-2-methyl-1H-indol-3-yl)acetic acid; Indometacina; Dolcidium; Elmetacin; Indomethine; Indorektal; Aconip; Catlep; Indoxen; Vonum; Indo-phlogont; Chibro-amuno; Rheumacin LA; 1H-Indole-3-acetic acid, 1-(4-chlorobenzoyl)-5-methoxy-2-methyl-; Osmosin; Tivorbex; 1-(p-Chlorobenzoyl)-5-methoxy-2-methylindole-3-acetic acid; CCRIS 3502; 2-[1-(4-chlorobenzoyl)-5-methoxy-2-methylindol-3-yl]acetic acid; HSDB 3101; 1-(4-Chlorobenzoyl)-5-methoxy-2-methyl-3-indoleacetic acid; NCI-C56144; MFCD00057095; Indometacin [INN]; Indomethacin (Indocid, Indocin); CHEMBL6; 1-(p-Chlorobenzoyl)-2-methyl-5-methoxyindole-3-acetic acid; IMN; Indole-3-acetic acid, 1-(p-chlorobenzoyl)-5-methoxy-2-methyl-; NSC-757061; 1-(4-Chlorobenzoyl)-5-methoxy-2-methyl-1H-indole-3-acetic acid; Indocin Sr; XXE1CET956; 2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl-1h-indol-3-yl}acetic acid; N-p-Chlorbenzoyl-5-methoxy-2-methylindole-3-acetic acid; MLS000069402; DTXSID9020740; (1-p-Chlorobenzoyl-5-methoxy-2-methylindol-3-yl)acetic acid; CHEBI:49662; 1-(p-Chlorobenzoyl)-2-methoxy-3-methyl-1H-indole-3-acetic Acid; Indomet 140; [1-(4-Chlorobenzoyl)-5-methoxy-2-methyl-1H-indol-3-yl]acetic acid; alpha-(1-(p-Chlorobenzoyl)-2-methyl-5-methoxy-3-indolyl)acetic acid; NSC-77541; CAS-53-86-1; 1-p-Cloro-benzoil-5-metoxi-2-metilindol-3-acido acetico; NCGC00015562-18; Indmethacine; Indomethancin; 1-(p-chlorobenzoyl)-5-methoxy-2-methyl-3-indolylacetic acid; Arthrexin; Bonidin; Bonidon; Indameth; Indomod; Miametan; SMR000058195; Indomo; Flexin continus; Hicin; Kwas 1-(p-chlorobenzoilo)-2-metylo-5-metoksy-3-indolilooctowy; Chrono-indicid; Chrono-indocid; Indometacyna [Polish]; Bonidon Gel; Indometicina [Spanish]; 2-[1-(4-chlorobenzoyl)-5-methoxy-2-methyl-1H-indol-3-yl]acetic acid; Dolcidium PL; Indo-Spray; Indolar SR; {1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl-1H-indol-3-yl}acetic acid; DTXCID50740; Indometacine [INN-French]; Indometacinum [INN-Latin]; Indometacina [INN-Spanish]; 1-(4-chlorobenzoyl)-5-methoxy-2-methylindole-3-acetic acid; 1-(p-Chlorobenzoyl)-5-methoxy-2-methyl-Indole-3-acetic acid; 2-[1-(4-chlorobenzoyl)-5-methoxy-2-methyl-indol-3-yl]acetic acid; 2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methylindol-3-yl}acetic acid; Indocin-SR; Indochron E-R; Indocin (TN); Aconip (TN); Indomethacin (USP); FLAM; Indocid (pharmaceutical); SR-01000000014; EINECS 200-186-5; Indomethacin & MAP-30; Indomethacin [USAN:USP]; UNII-XXE1CET956; BRN 0497341; Indocollyre; Indonol; Innamit; Inteban; (1-(4-CHLOROBENZOYL)-5-METHOXY-2-METHYL-1H-INDOL-3-YL)ACETIC ACID; 2-{1-((4-chlorophenyl)carbonyl)-5-methoxy-2-methylindol-3-yl}acetic acid; 4kyk; Indomethacin,(S); Prestwick_597; Opera_ID_56; Spectrum_000919; Tocris-1708; 1z9h; 1-(p-Chlorbenzoyl)-5-methoxy-2-methylindol-3-essigsaeure [German]; 1-p-Cloro-benzoil-5-metoxi-2-metilindol-3-acido acetico [Spanish]; Prestwick0_000272; Prestwick1_000272; Prestwick2_000272; Prestwick3_000272; Spectrum2_000970; Spectrum3_000468; Spectrum4_000018; Spectrum5_000868; INDOMETACIN [JAN]; INDOMETHACIN [MI]; Lopac-I-7378; Kwas 1-(p-chlorobenzoilo)-2-metylo-5-metoksy-3-indolilooctowy [Polish]; MolMap_000032; UPCMLD-DP023; EC 200-186-5; I 7378; Indometacin (JP17/INN); INDOMETHACIN [HSDB]; INDOMETHACIN [USAN]; SCHEMBL9300; INDOMETACIN [MART.]; INDOMETHACIN [VANDF]; Lopac0_000692; Oprea1_686105; BSPBio_000144; BSPBio_001149; BSPBio_002176; INDOMETACIN [WHO-DD]; INDOMETACIN [WHO-IP]; Indomethacine impurity mixture; KBioGR_000395; KBioGR_000489; KBioSS_000489; KBioSS_001399; 5-22-05-00239 (Beilstein Handbook Reference); MLS001074194; MLS006011845; BIDD:GT0132; DivK1c_000271; INDOMETHACIN [USP-RS]; SPECTRUM1500350; SPBio_000979; SPBio_002363; BPBio1_000160; GTPL1909; Indomethacin, >=99% (TLC); UPCMLD-DP023:001; BDBM17638; CGIGDMFJXJATDK-UHFFFAOYSA-; HMS500N13; KBio1_000271; KBio2_000489; KBio2_001399; KBio2_003057; KBio2_003967; KBio2_005625; KBio2_006535; KBio3_000897; KBio3_000898; KBio3_001396; 1-(p-Chlorbenzoyl)-5-methoxy-2-methylindol-3-essigsaeure; Indomethacin - CAS 53-86-1; NINDS_000271; Bio2_000405; Bio2_000885; HMS1362I11; HMS1568H06; HMS1792I11; HMS1920F21; HMS1990I11; HMS2089N19; HMS2091N09; HMS2095H06; HMS2231J10; HMS3262K05; HMS3268A14; HMS3374F07; HMS3403I11; HMS3414N13; HMS3430L03; HMS3649K17; HMS3655O04; HMS3678N11; HMS3712H06; HMS3747K21; HMS3884E08; INDOMETACIN [EP MONOGRAPH]; INDOMETHACIN [ORANGE BOOK]; Indomethacin-d4(chlorobenzoyl-d4); Pharmakon1600-01500350; ZINC601283; ACT02579; BCP18951; Indomethacin, >=99.0% (TLC); INDOMETHACIN [USP MONOGRAPH]; Tox21_113109; Tox21_201791; Tox21_300266; Tox21_500692; AC-532; CCG-40186; HB4422; INDOMETACINUM [WHO-IP LATIN]; NSC757061; s1723; STL257874; AKOS000592893; Tox21_113109_1; AT13679; DB00328; Indometacin 1.0 mg/ml in Acetonitrile; KS-5255; LP00692; NSC 757061; SDCCGSBI-0050670.P005; IDI1_000271; IDI1_002160; NCGC00015562-01; NCGC00015562-02; NCGC00015562-03; NCGC00015562-04; NCGC00015562-05; NCGC00015562-06; NCGC00015562-07; NCGC00015562-08; NCGC00015562-09; NCGC00015562-10; NCGC00015562-11; NCGC00015562-12; NCGC00015562-13; NCGC00015562-14; NCGC00015562-15; NCGC00015562-16; NCGC00015562-17; NCGC00015562-19; NCGC00015562-20; NCGC00015562-21; NCGC00015562-22; NCGC00015562-24; NCGC00015562-25; NCGC00015562-40; NCGC00024135-02; NCGC00024135-04; NCGC00024135-05; NCGC00024135-06; NCGC00024135-07; NCGC00024135-08; NCGC00024135-09; NCGC00024135-10; NCGC00024135-11; NCGC00024135-12; NCGC00024135-13; NCGC00024135-14; NCGC00024135-15; NCGC00254075-01; NCGC00259340-01; NCGC00261377-01; BI166166; BP-30207; HY-14397; NCI60_041708; SBI-0050670.P004; ACEMETACIN IMPURITY B [EP IMPURITY]; DB-052413; AB00052022; EU-0100692; FT-0603227; I0655; SW196768-5; EN300-16807; Indomethacin, meets USP testing specifications; BIM-0050670.0001; C01926; D00141; S00108; AB00052022-20; AB00052022-21; AB00052022_23; AB00052022_24; L000959; Q409231; Indomethacin, Antibiotic for Culture Media Use Only; Q-201239; SR-01000000014-2; SR-01000000014-4; SR-01000000014-6; 1-(4-Chlorobenzoyl)-5-methoxy-2-met hyl-1H-indole; BRD-K57222227-001-06-1; BRD-K57222227-001-18-6; BRD-K57222227-001-27-7; SR-01000000014-10; SR-01000000014-16; Z56784896; 1-p-chlorobenzoyl-2-methyl-5-methoxyindol-3-acetic acid; 1-(p-chlorobenzoyl)-2-methyl-5-methoxy-3-indoleacetic acid; 1-(p-chlorobenzoyl)-5-methoxy-2-methyl-3-indoleacetic acid; 1-(p-Chlorobenzoyl)-5-methoxy-2-methylindol-3-acetic acid; 1-(4-chloro-benzoyl)-5-methoxy-2-methyl-3-indolyl-acetic acid; 1-(4-Chlorobenzoyl)-2-methyl-5-methoxyindole-3-acetic acid; 1-(p-Chlorobenzoyl)-2-methyl-5-methoxy-3-indole-acetic acid; 1-(p-chlorobenzoyl)-2-methyl-5-methoxy-3-indolylacetic acid; 1-(p-chlorobenzoyl)-5-methoxy-2-methyl-3-indol acetic acid; Indomethacin, European Pharmacopoeia (EP) Reference Standard; N-(p-Chlorobenzoyl)-2-methyl-5-methoxy-3-indolylacetic acid; Indomethacin, United States Pharmacopeia (USP) Reference Standard; .alpha.-(1-(p-Chlorobenzoyl)-2-methyl-5-methoxy-3-indolyl)acetic acid; [1-(4-Chlorobenzoyl)-5-methoxy-2-methyl-1H-indol-3-yl]acetic acid #; 1-(4-Chlorobenzoyl)-5-methoxy-2-methyl-1H-indole-3-acetic acid & MAP-30; 1H-Indole-3-acetic acid, 1-(4-chlorobenzoyl)-5-methoxy-2-methyl- (9CI); 2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2 methylindol-3-yl}acetic acid; 2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2methylindol-3-yl}acetic acid; Indole-3-acetic acid, 1-(p-chlorobenzoyl)-5-methoxy-2-methyl- (8CI); Indomethacin, Pharmaceutical Secondary Standard; Certified Reference Material
|