NPs Basic Information
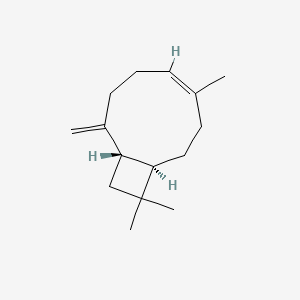
|
Name |
Isocaryophyllene
|
| Molecular Formula | C15H24 | |
| IUPAC Name* |
(1R,4Z,9S)-4,11,11-trimethyl-8-methylidenebicyclo[7.2.0]undec-4-ene
|
|
| SMILES |
C/C/1=C/CCC(=C)[C@H]2CC([C@@H]2CC1)(C)C
|
|
| InChI |
InChI=1S/C15H24/c1-11-6-5-7-12(2)13-10-15(3,4)14(13)9-8-11/h6,13-14H,2,5,7-10H2,1,3-4H3/b11-6-/t13-,14-/m1/s1
|
|
| InChIKey |
NPNUFJAVOOONJE-FLFDDASRSA-N
|
|
| Synonyms |
Isocaryophyllene; 118-65-0; (-)-ISOCARYOPHYLLENE; gamma-caryophyllene; Isocaryophyllene (80per cent); 87-44-5; (1R,4Z,9S)-4,11,11-trimethyl-8-methylidenebicyclo[7.2.0]undec-4-ene; NRY8I0KNIR; CHEBI:5993; beta-Caryophyllen; Cis-caryophyllene; Bicyclo(7.2.0)undec-4-ene, 4,11,11-trimethyl-8-methylene-, (1R,4Z,9S)-; g-Caryophyllene; NSC 11906; .gamma.-Caryophyllene; .beta.-cis-Caryophyllene; Caryophyllene (VAN); BHW853AU9H; UNII-NRY8I0KNIR; beta-Caryophyllene (natural); E-Caryophyllene; ss-Caryophyllene; Bicyclo(7.2.0)undec-4-ene, 4,11,11-trimethyl-8-methylene-, (1R,4E,9S)-; Bicyclo(7.2.0)undec-4-ene, 4,11,11-trimethyl-8-methylene-, (1R-(1R*,4E,9S*))-; EINECS 201-746-1; EINECS 204-267-6; (-)--caryophyllene; (-)-E-Caryophyllene; AI3-36121; UNII-BHW853AU9H; 4,11,11-Trimethyl-8-methylenebicyclo[7.2.0]undec-4-ene #; CCRIS 8094; CHEMBL448700; (1R,9S,E)-4,11,11-trimethyl-8-methylenebicyclo[7.2.0]undec-4-ene; 2-Methylene-6,10,10-trimethylbicyclo(7.2.0)undec-5-ene; DTXSID10881246; CARYOPHYLLENE ISOCARYOPHYLLENE; ZINC30726969; (-)-Caryophyllene sum of enantiomers; LMPR0103120002; 4,11,11-Trimethyl-8-methylenebicyclo(7.2.0)undec-4-ene, (1R-(1R*,4E,9S))-; BS-48823; CARYOPHYLLENE ISOCARYOPHYLLENE [MI]; CARYOPHYLLENE, ALPHA + BETA MIXT.; C09691; E75762; Q27106968; (1beta,9alpha)-4,11,11-Trimethyl-8-methylenebicyclo[7.2.0]undeca-4-ene; (1R-(1R*,4Z,9S*))-4,11,11-Trimethyl-8-methylenebicyclo(7.2.0)undec-4-ene
|
|
| CAS | 87-44-5 | |
| PubChem CID | 5281522 | |
| ChEMBL ID | CHEMBL448700 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 204.35 | ALogp: | 4.4 |
| HBD: | 0 | HBA: | 0 |
| Rotatable Bonds: | 0 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 0.0 | Aromatic Rings: | 2 |
| Heavy Atoms: | 15 | QED Weighted: | 0.478 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.576 | MDCK Permeability: | 0.00001990 |
| Pgp-inhibitor: | 0.072 | Pgp-substrate: | 0 |
| Human Intestinal Absorption (HIA): | 0.005 | 20% Bioavailability (F20%): | 0.977 |
| 30% Bioavailability (F30%): | 0.019 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.566 | Plasma Protein Binding (PPB): | 90.63% |
| Volume Distribution (VD): | 2.19 | Fu: | 13.22% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.458 | CYP1A2-substrate: | 0.796 |
| CYP2C19-inhibitor: | 0.345 | CYP2C19-substrate: | 0.891 |
| CYP2C9-inhibitor: | 0.327 | CYP2C9-substrate: | 0.842 |
| CYP2D6-inhibitor: | 0.063 | CYP2D6-substrate: | 0.897 |
| CYP3A4-inhibitor: | 0.143 | CYP3A4-substrate: | 0.288 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 6.767 | Half-life (T1/2): | 0.259 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.033 | Human Hepatotoxicity (H-HT): | 0.217 |
| Drug-inuced Liver Injury (DILI): | 0.14 | AMES Toxicity: | 0.007 |
| Rat Oral Acute Toxicity: | 0.059 | Maximum Recommended Daily Dose: | 0.523 |
| Skin Sensitization: | 0.517 | Carcinogencity: | 0.241 |
| Eye Corrosion: | 0.449 | Eye Irritation: | 0.855 |
| Respiratory Toxicity: | 0.485 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC001826 | 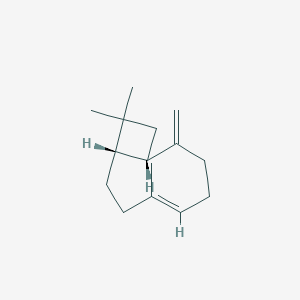 |
1.000 | D0L2LS | 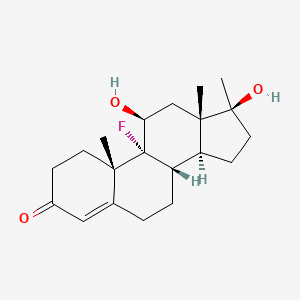 |
0.262 | ||
| ENC001663 | 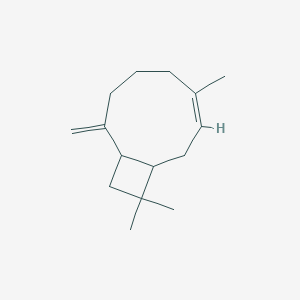 |
0.673 | D0D2VS | 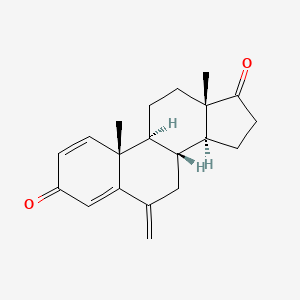 |
0.259 | ||
| ENC002199 | 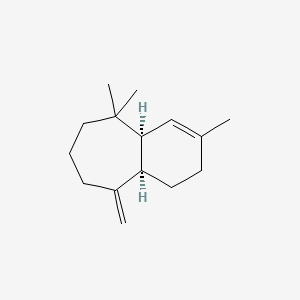 |
0.519 | D0K0EK | 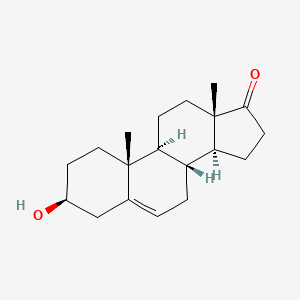 |
0.250 | ||
| ENC002652 | 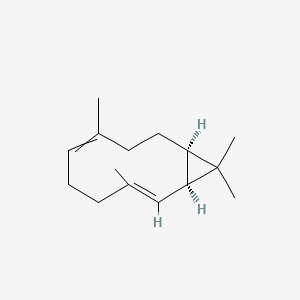 |
0.519 | D06CGB |  |
0.247 | ||
| ENC001469 |  |
0.491 | D0A2AJ |  |
0.247 | ||
| ENC001297 |  |
0.446 | D0Z1XD |  |
0.244 | ||
| ENC002990 |  |
0.414 | D0F2AK |  |
0.241 | ||
| ENC001316 |  |
0.414 | D0C7JF | 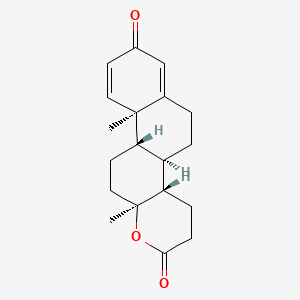 |
0.241 | ||
| ENC000800 | 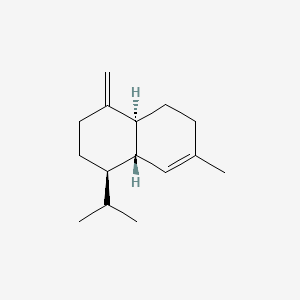 |
0.390 | D00ZFP | 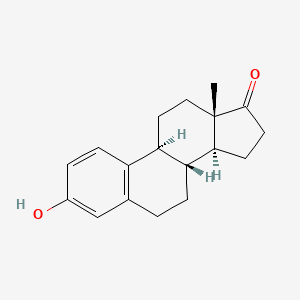 |
0.241 | ||
| ENC001279 | 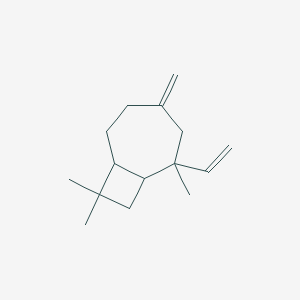 |
0.373 | D04ATM |  |
0.236 | ||