NPs Basic Information
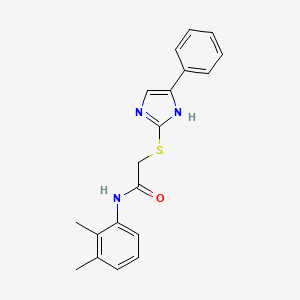
|
Name |
N-(2,3-Dimethylphenyl)-2-((4-phenyl-1H-imidazol-2-yl)thio)acetamide
|
| Molecular Formula | C19H19N3OS | |
| IUPAC Name* |
N-(2,3-dimethylphenyl)-2-[(5-phenyl-1H-imidazol-2-yl)sulfanyl]acetamide
|
|
| SMILES |
CC1=C(C(=CC=C1)NC(=O)CSC2=NC=C(N2)C3=CC=CC=C3)C
|
|
| InChI |
InChI=1S/C19H19N3OS/c1-13-7-6-10-16(14(13)2)21-18(23)12-24-19-20-11-17(22-19)15-8-4-3-5-9-15/h3-11H,12H2,1-2H3,(H,20,22)(H,21,23)
|
|
| InChIKey |
MVBJAPWJNOFGMS-UHFFFAOYSA-N
|
|
| Synonyms |
312278-95-8; N-(2,3-Dimethylphenyl)-2-((4-phenyl-1H-imidazol-2-yl)thio)acetamide; N-(2,3-dimethylphenyl)-2-[(4-phenyl-1H-imidazol-2-yl)thio]acetamide; Cambridge id 5927802; Oprea1_434997; Oprea1_495745; CHEMBL1398569; HMS1807D17; ZINC4565091; STK735635; ZINC00051853; AKOS000568349; N-(2,3-Dimethyl-phenyl)-2-(4-phenyl-1H-imidazol-2-ylsulfanyl)-acetamide; N-(2,3-dimethylphenyl)-2-[(5-phenyl-1H-imidazol-2-yl)sulfanyl]acetamide; NCGC00102580-01; EU-0018308; AB00101070-01; SR-01000519341; SR-01000519341-1; Acetamide, 2-(4-phenyl-2-imidazolylthio)-N-(2,3-dimethylphenyl)-; N-(2,3-Dimethylphenyl)-2-[(4-phenyl-1H-imidazol-2-yl)sulfanyl]acetamide; N-(2,3-Dimethylphenyl)-2-[(4-phenyl-1H-imidazol-2-yl)sulfanyl]acetamide #
|
|
| CAS | NA | |
| PubChem CID | 593949 | |
| ChEMBL ID | CHEMBL1398569 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 337.4 | ALogp: | 4.2 |
| HBD: | 2 | HBA: | 3 |
| Rotatable Bonds: | 5 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 83.1 | Aromatic Rings: | 3 |
| Heavy Atoms: | 24 | QED Weighted: | 0.648 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.87 | MDCK Permeability: | 0.00002360 |
| Pgp-inhibitor: | 0.065 | Pgp-substrate: | 0.029 |
| Human Intestinal Absorption (HIA): | 0.006 | 20% Bioavailability (F20%): | 0.002 |
| 30% Bioavailability (F30%): | 0.001 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.388 | Plasma Protein Binding (PPB): | 99.42% |
| Volume Distribution (VD): | 0.466 | Fu: | 0.85% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.944 | CYP1A2-substrate: | 0.674 |
| CYP2C19-inhibitor: | 0.962 | CYP2C19-substrate: | 0.103 |
| CYP2C9-inhibitor: | 0.933 | CYP2C9-substrate: | 0.857 |
| CYP2D6-inhibitor: | 0.771 | CYP2D6-substrate: | 0.53 |
| CYP3A4-inhibitor: | 0.924 | CYP3A4-substrate: | 0.536 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 10.256 | Half-life (T1/2): | 0.742 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.199 | Human Hepatotoxicity (H-HT): | 0.366 |
| Drug-inuced Liver Injury (DILI): | 0.958 | AMES Toxicity: | 0.922 |
| Rat Oral Acute Toxicity: | 0.076 | Maximum Recommended Daily Dose: | 0.273 |
| Skin Sensitization: | 0.947 | Carcinogencity: | 0.12 |
| Eye Corrosion: | 0.004 | Eye Irritation: | 0.625 |
| Respiratory Toxicity: | 0.948 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC001388 | 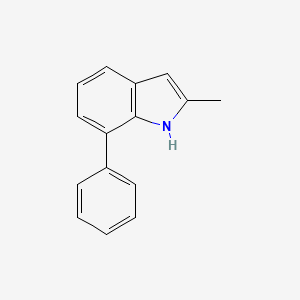 |
0.398 | D05FTJ | 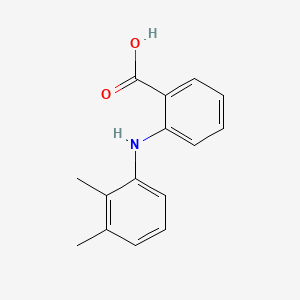 |
0.451 | ||
| ENC001109 |  |
0.363 | D05UWI | 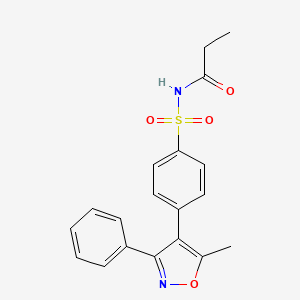 |
0.365 | ||
| ENC003390 | 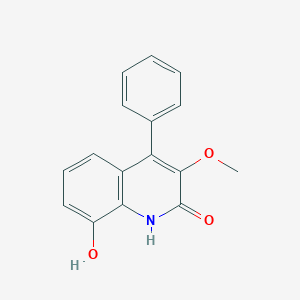 |
0.355 | D09VXM | 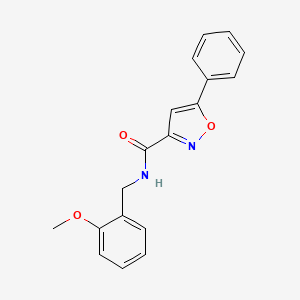 |
0.347 | ||
| ENC000321 | 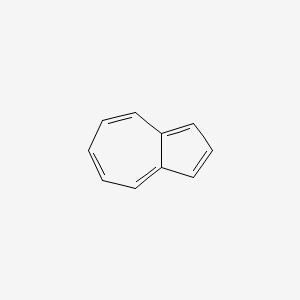 |
0.338 | D0Y7EM | 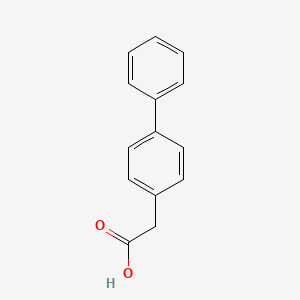 |
0.337 | ||
| ENC001352 | 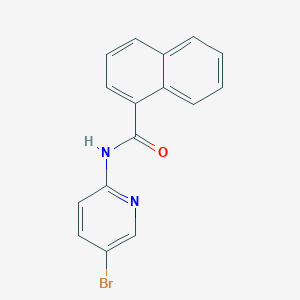 |
0.337 | D0J6WW | 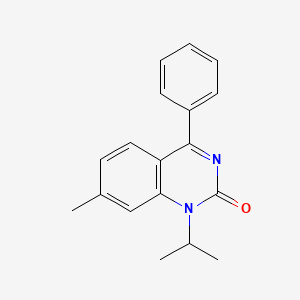 |
0.333 | ||
| ENC001307 | 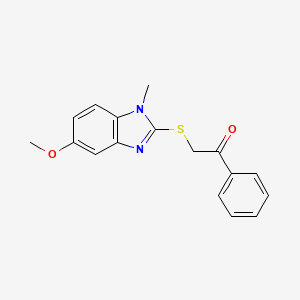 |
0.333 | D0YB1G | 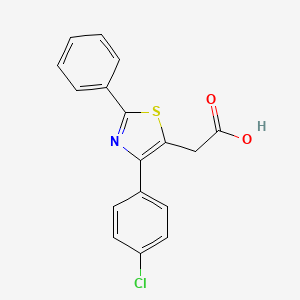 |
0.333 | ||
| ENC005445 | 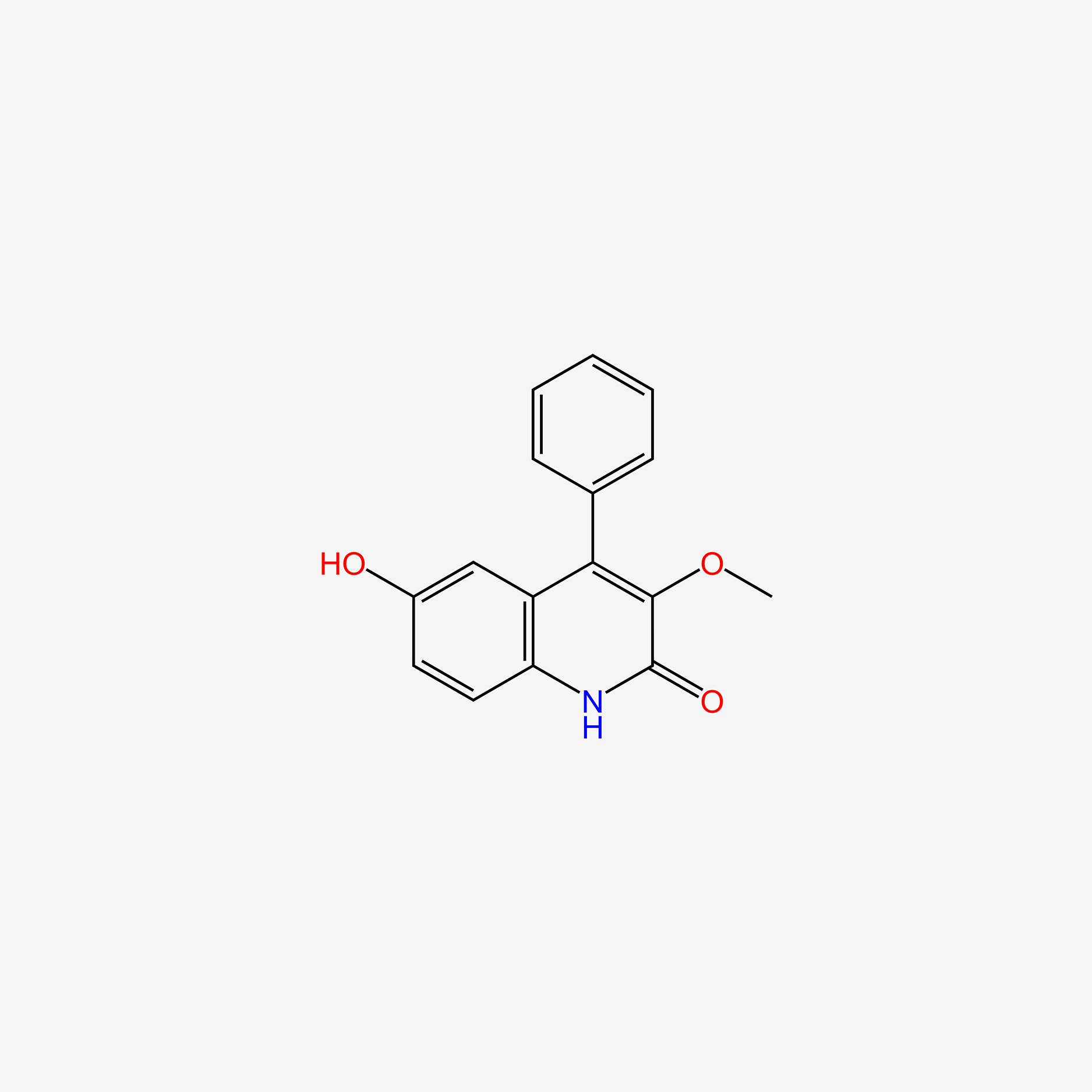 |
0.326 | D09ZXR | 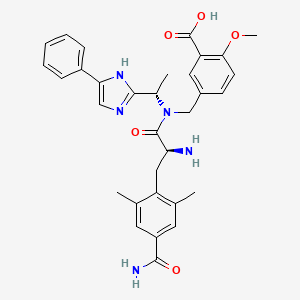 |
0.331 | ||
| ENC000732 | 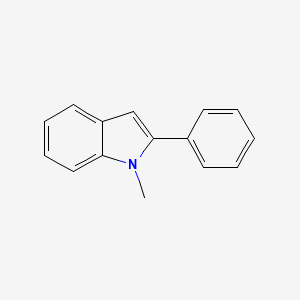 |
0.318 | D0M9DC | 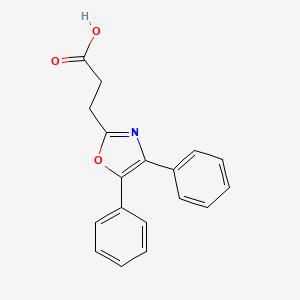 |
0.330 | ||
| ENC003342 | 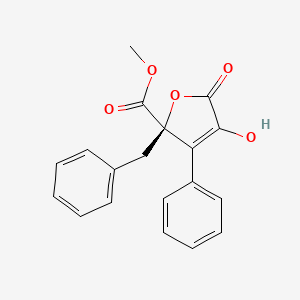 |
0.317 | D0P3JU | 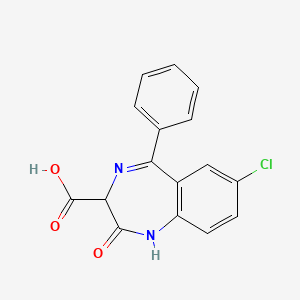 |
0.323 | ||
| ENC005446 | 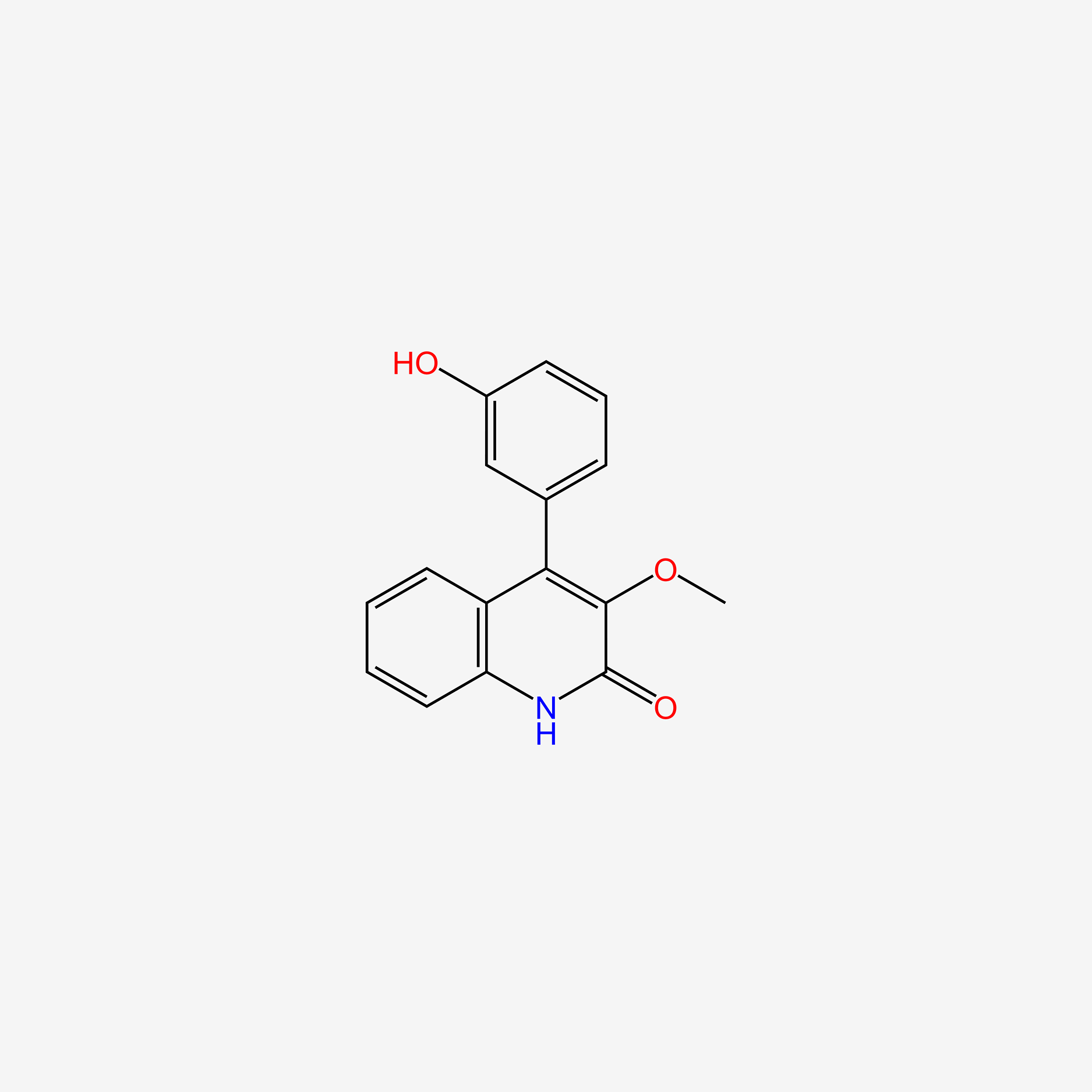 |
0.313 | D0L6DA | 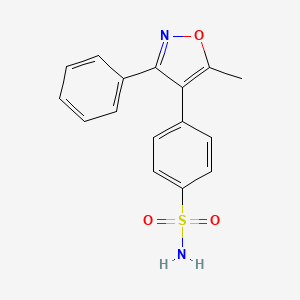 |
0.323 | ||