NPs Basic Information
|
Name |
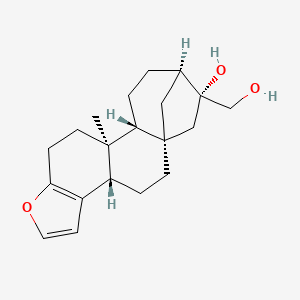
Cafestol
|
| Molecular Formula | C20H28O3 | |
| IUPAC Name* |
(1S,4S,12S,13R,16R,17R)-17-(hydroxymethyl)-12-methyl-8-oxapentacyclo[14.2.1.01,13.04,12.05,9]nonadeca-5(9),6-dien-17-ol
|
|
| SMILES |
C[C@@]12CCC3=C([C@H]1CC[C@]45[C@H]2CC[C@H](C4)[C@](C5)(CO)O)C=CO3
|
|
| InChI |
InChI=1S/C20H28O3/c1-18-7-5-16-14(6-9-23-16)15(18)4-8-19-10-13(2-3-17(18)19)20(22,11-19)12-21/h6,9,13,15,17,21-22H,2-5,7-8,10-12H2,1H3/t13-,15-,17+,18-,19+,20+/m1/s1
|
|
| InChIKey |
DNJVYWXIDISQRD-HWUKTEKMSA-N
|
|
| Synonyms |
cafestol; 469-83-0; cafesterol; CCRIS 1518; AC465T6Q6W; CHEBI:3291; (3bS,5aS,7R,8R,10aR,10bS)-7-(hydroxymethyl)-10b-methyl-3b,4,5,6,7,8,9,10,10a,10b,11,12-dodecahydro-5a,8-methanocyclohepta[5,6]naphtho[2,1-b]furan-7-ol; UNII-AC465T6Q6W; SR-05000002204; (-)-cafestol; CAFESTOL [MI]; SCHEMBL93865; CHEMBL1407645; DTXSID3040986; HY-N6257; ZINC4097876; MFCD01075769; AKOS032949533; CCG-208556; 5a,8-Methano-5aH-cyclohepta(5,6)naphtho(2,1-b)furan-7-methanol, 3b,4,5,6,7,8,9,10,10a,10b,11,12-dodecahydro-7-hydroxy-10b-methyl-, (3bS,5aS,7R,8R,10aR,10bS)-; 5a,8-Methano-5aH-cyclohepta(5,6)naphtho(2,1-b)furan-7-methanol, 3b,4,5,6,7,8,9,10,10a,10b,11,12-dodecahydro-7-hydroxy-10b-methyl-, (3bS-(3balpha,5abeta,7beta,8beta,10aalpha,10bbeta))-; CS-0032793; C09066; E80644; Q423829; SR-05000002204-2; SR-05000002204-3; (1S,4S,12S,13R,16R,17R)-17-(hydroxymethyl)-12-methyl-8-oxapentacyclo[14.2.1.0(1,13).0(4,12).0(5,9)]nonadeca-5(9),6-dien-17-ol; (1S,4S,12S,13R,16R,17R)-17-(hydroxymethyl)-12-methyl-8-oxapentacyclo[14.2.1.01,13.04,12.05,9]nonadeca-5(9),6-dien-17-ol; 5A,8-METHANO-5AH-CYCLOHEPTA(5,6)NAPHTHO(2,1-B)FURAN-7-METHANOL, 3B,4,5,6,7,8,9,10,10A,10B,11,12-DODECAHYDRO-7-HYDROXY-10B-METHYL-, (3BS-(3B.ALPHA.,5A.BETA.,7.BETA.,8.BETA.,10A.ALPHA.,10B.BETA.))-
|
|
| CAS | 469-83-0 | |
| PubChem CID | 108052 | |
| ChEMBL ID | CHEMBL1407645 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 316.4 | ALogp: | 3.3 |
| HBD: | 2 | HBA: | 3 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 53.6 | Aromatic Rings: | 5 |
| Heavy Atoms: | 23 | QED Weighted: | 0.81 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -5.223 | MDCK Permeability: | 0.00000795 |
| Pgp-inhibitor: | 0.009 | Pgp-substrate: | 0.088 |
| Human Intestinal Absorption (HIA): | 0.006 | 20% Bioavailability (F20%): | 0.453 |
| 30% Bioavailability (F30%): | 0.993 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.161 | Plasma Protein Binding (PPB): | 95.48% |
| Volume Distribution (VD): | 1.279 | Fu: | 2.95% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.041 | CYP1A2-substrate: | 0.335 |
| CYP2C19-inhibitor: | 0.04 | CYP2C19-substrate: | 0.759 |
| CYP2C9-inhibitor: | 0.147 | CYP2C9-substrate: | 0.189 |
| CYP2D6-inhibitor: | 0.066 | CYP2D6-substrate: | 0.64 |
| CYP3A4-inhibitor: | 0.942 | CYP3A4-substrate: | 0.277 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 13.284 | Half-life (T1/2): | 0.442 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.186 | Human Hepatotoxicity (H-HT): | 0.57 |
| Drug-inuced Liver Injury (DILI): | 0.171 | AMES Toxicity: | 0.016 |
| Rat Oral Acute Toxicity: | 0.903 | Maximum Recommended Daily Dose: | 0.893 |
| Skin Sensitization: | 0.456 | Carcinogencity: | 0.591 |
| Eye Corrosion: | 0.004 | Eye Irritation: | 0.024 |
| Respiratory Toxicity: | 0.97 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC002608 | 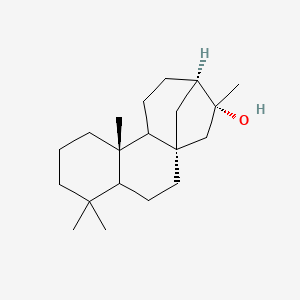 |
0.393 | D0U3GL | 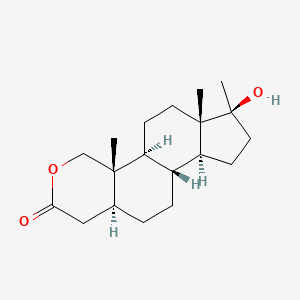 |
0.283 | ||
| ENC005747 | 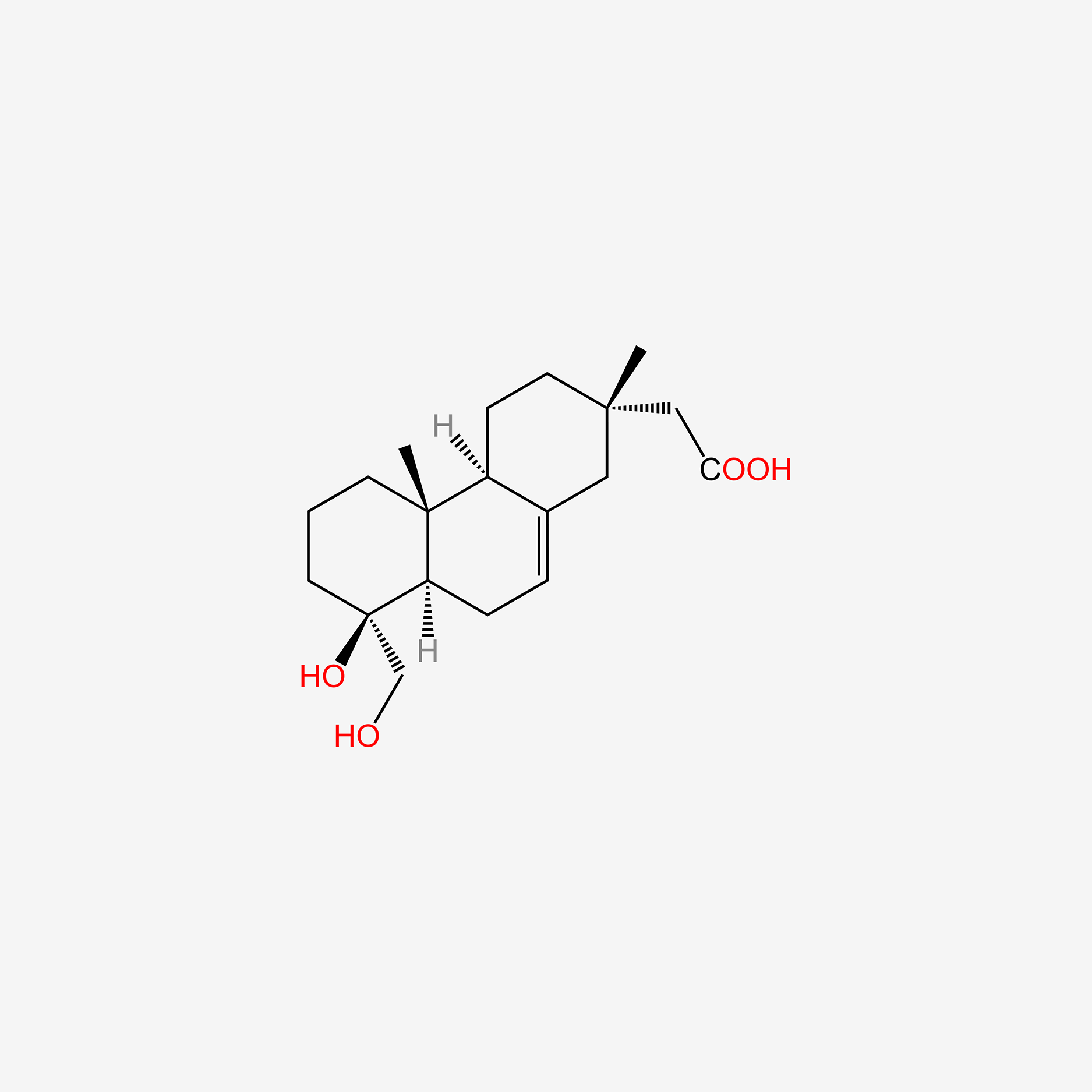 |
0.330 | D0Q6NZ | 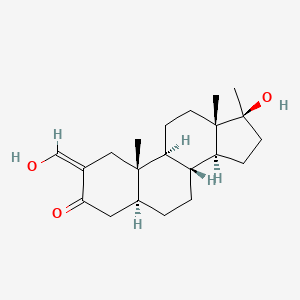 |
0.282 | ||
| ENC003145 | 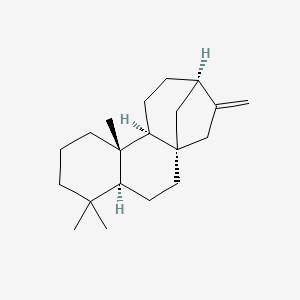 |
0.312 | D06NXY | 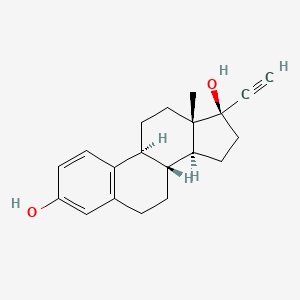 |
0.280 | ||
| ENC005141 | 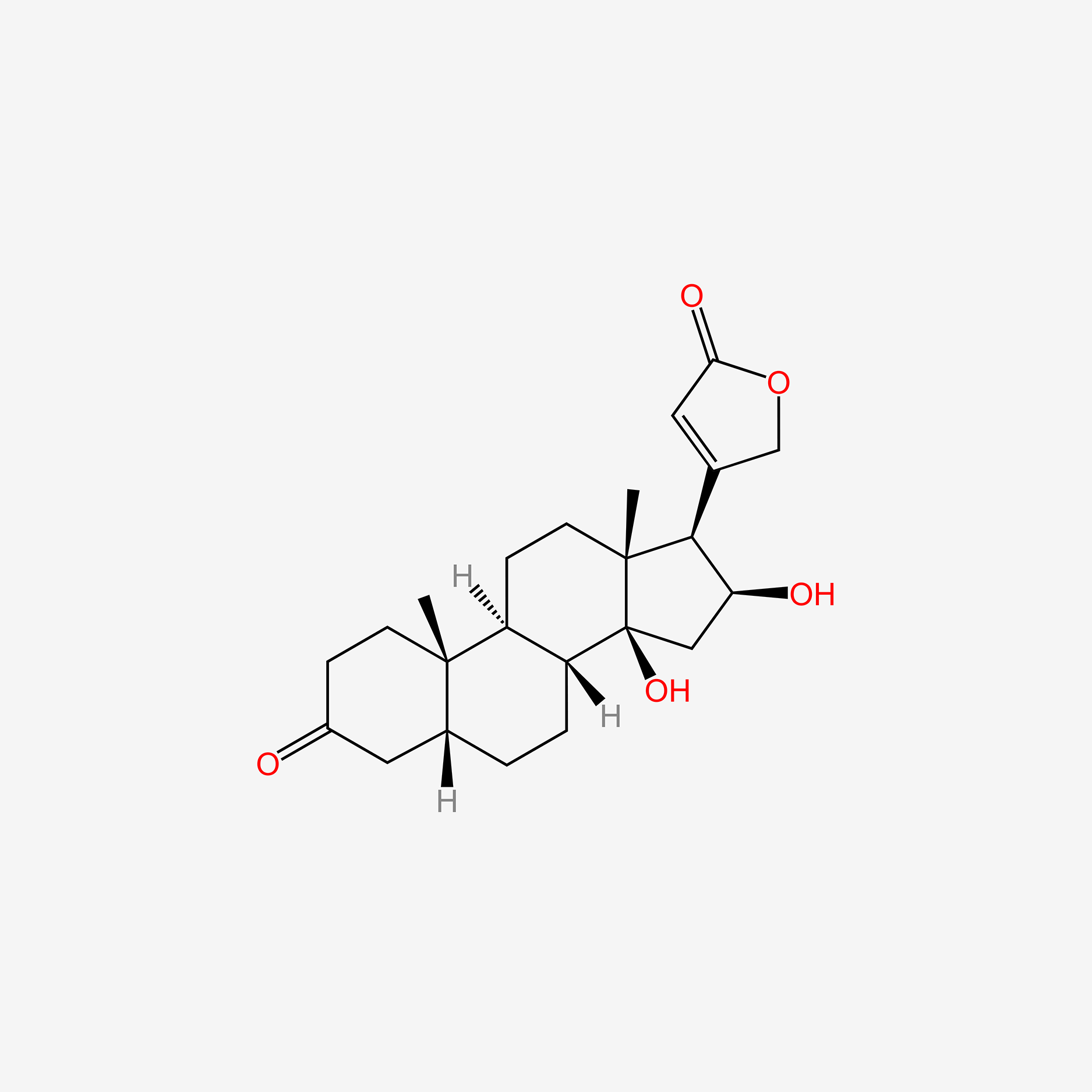 |
0.309 | D08QKJ | 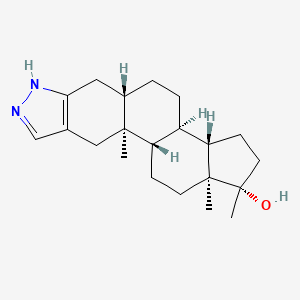 |
0.276 | ||
| ENC004729 | 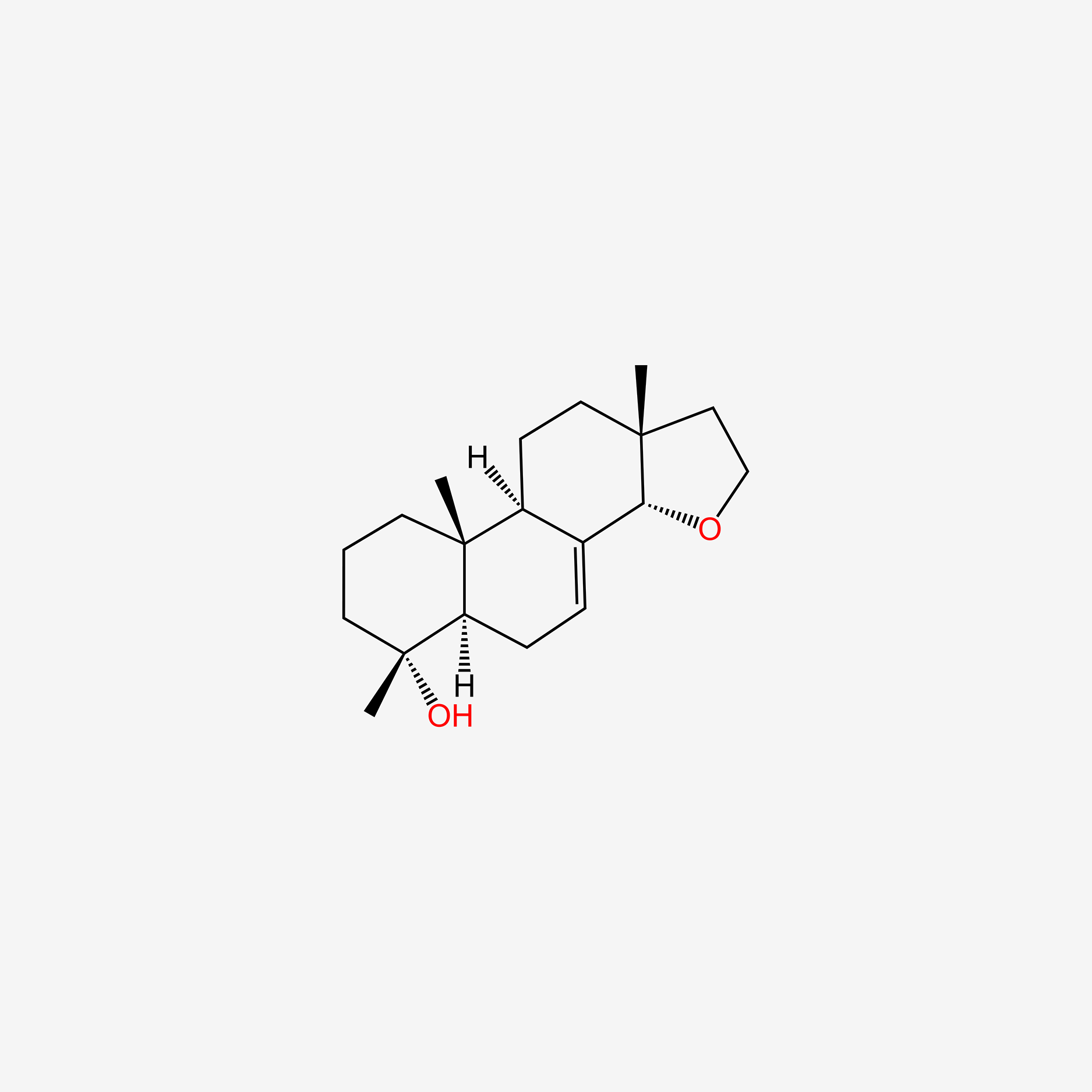 |
0.302 | D04DJN | 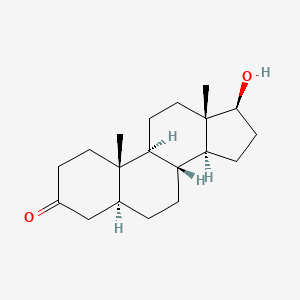 |
0.276 | ||
| ENC002305 | 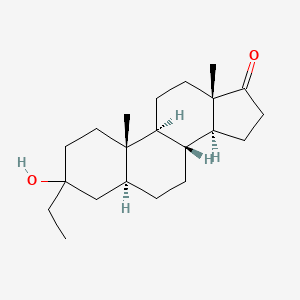 |
0.300 | D0Z1XD |  |
0.270 | ||
| ENC002216 |  |
0.292 | D0KR5B |  |
0.269 | ||
| ENC005144 |  |
0.286 | D08QMX | 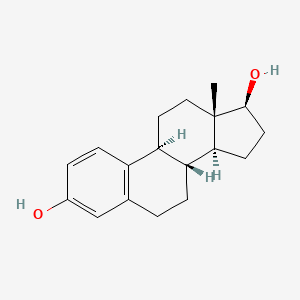 |
0.268 | ||
| ENC005146 | 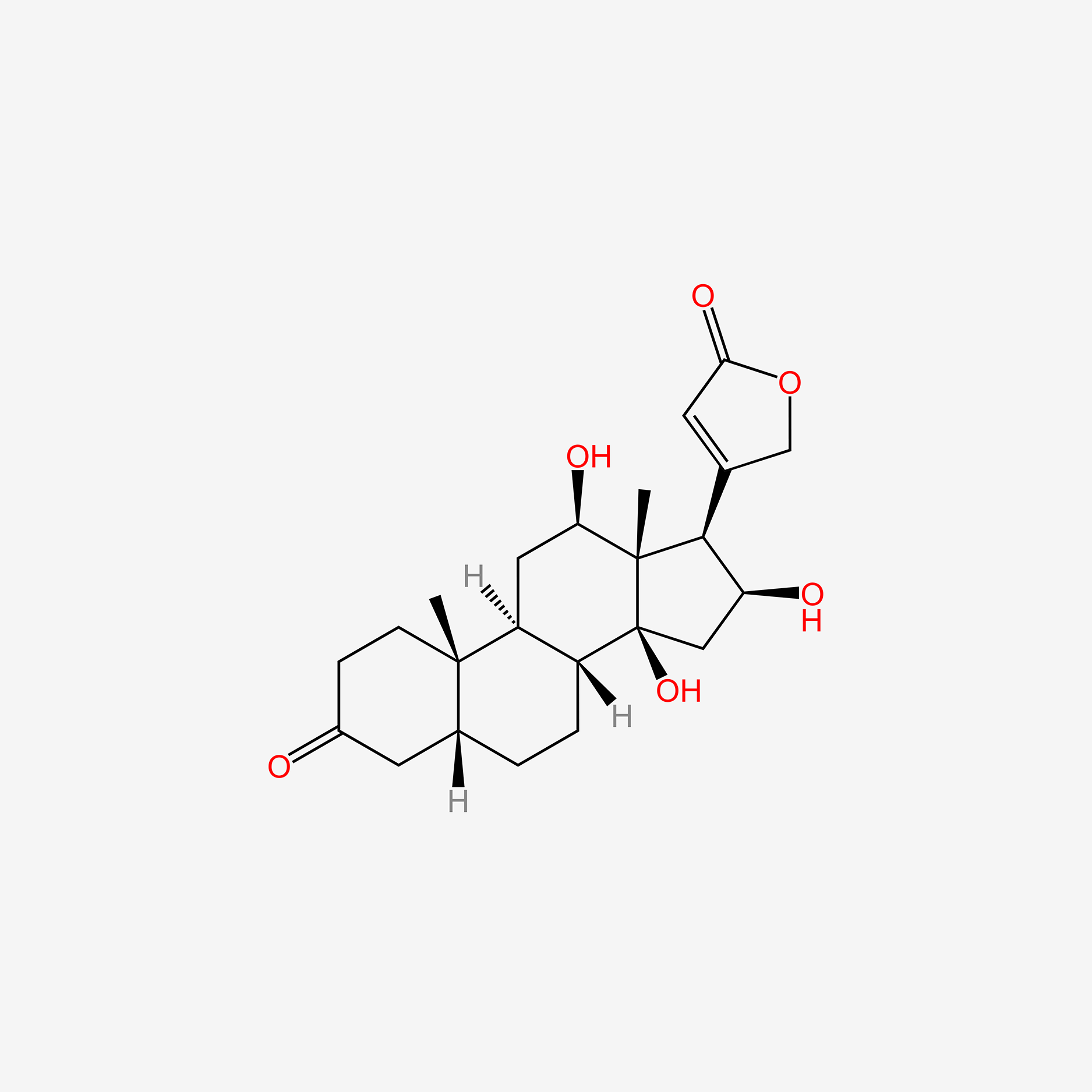 |
0.281 | D0R7JT | 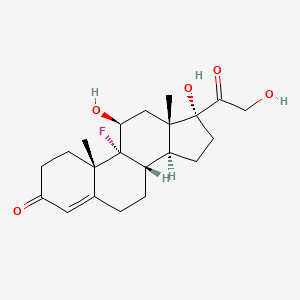 |
0.264 | ||
| ENC002934 | 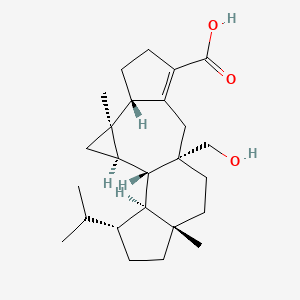 |
0.277 | D06XMU |  |
0.263 | ||