NPs Basic Information
|
Name |
antimycin A1
|
| Molecular Formula | C28H40N2O9 | |
| IUPAC Name* |
[(2R,3S,6S,7R,8R)-3-[(3-formamido-2-hydroxybenzoyl)amino]-8-hexyl-2,6-dimethyl-4,9-dioxo-1,5-dioxonan-7-yl] 3-methylbutanoate
|
|
| SMILES |
CCCCCC[C@@H]1[C@H]([C@@H](OC(=O)[C@H]([C@H](OC1=O)C)NC(=O)C2=C(C(=CC=C2)NC=O)O)C)OC(=O)CC(C)C
|
|
| InChI |
InChI=1S/C28H40N2O9/c1-6-7-8-9-11-20-25(39-22(32)14-16(2)3)18(5)38-28(36)23(17(4)37-27(20)35)30-26(34)19-12-10-13-21(24(19)33)29-15-31/h10,12-13,15-18,20,23,25,33H,6-9,11,14H2,1-5H3,(H,29,31)(H,30,34)/t17-,18+,20-,23+,25+/m1/s1
|
|
| InChIKey |
UIFFUZWRFRDZJC-SBOOETFBSA-N
|
|
| Synonyms |
Antimycin A; Antimycin A1b; Antipiricullin; Fintrol; Virosin; antimycin A1; 1397-94-0; Antimycin-A; Caswell No. 052B; CCRIS 924; HSDB 6417; 116095-18-2; EPA Pesticide Chemical Code 006314; 75G3NMU1TS; CHEMBL211501; CHEBI:22584; (2R,3S,6S,7R,8R)-3-[(3-formamido-2-hydroxybenzoyl)amino]-8-hexyl-2,6-dimethyl-4,9-dioxo-1,5-dioxonan-7-yl 3-methylbutanoate; [(2R,3S,6S,7R,8R)-3-[(3-formamido-2-hydroxybenzoyl)amino]-8-hexyl-2,6-dimethyl-4,9-dioxo-1,5-dioxonan-7-yl] 3-methylbutanoate; [(2r,3s,6s,7r,8r)-3-[(3-Formamido-2-Oxidanyl-Phenyl)carbonylamino]-8-Hexyl-2,6-Dimethyl-4,9-Bis(Oxidanylidene)-1,5-Dioxonan-7-Yl] 3-Methylbutanoate; Butanoic acid, 3-methyl-, 3-((3-(formylamino)-2-hydroxybenzoyl)amino)-8-hexyl-2,6-dimethyl-4,9-dioxo-1,5-dioxonan-7-yl ester, (2R-(2R*,3S*,6S*,7R*,8R*))-; SR-05000002233; UNII-75G3NMU1TS; UNII-8S75R39Y6J; AWB; SCHEMBL218354; CHEBI:2762; DTXSID3058668; 8S75R39Y6J; AIDS032154; ZINC5224254; BDBM50191588; AKOS015889205; BCP9000305; CCG-208457; NCGC00017338-02; NCGC00017338-03; NCGC00017338-04; NCGC00017338-05; NCGC00142516-01; NCGC00142516-02; NCGC00142516-03; HY-107406; CS-0028420; SR-05000002233-2; SR-05000002233-3; [(2R,3S,6S,7R,8R)-3-[(3-formamido-2-hydroxy-benzoyl)amino]-8-hexyl-2,6-dimethyl-4,9-dioxo-1,5-dioxonan-7-yl] 3-methylbutanoate; 2(or 3)-Methylbutanoic acid, (2R,3S,6S,7R,8R)-3-[[3-(formylamino)-2-hydroxybenzoyl]amino]-8-hexyl-2,6-dimethyl-4,9-dioxo-1,5-dioxonan-7-yl ester; 3-Methylbutanoic acid 3-[[3-(formylamino)-2-hydroxybenzoyl]amino]-8-hexyl-2,6-dimethyl-4,9-dioxo-1,5-dioxonan-7-yl ester; BUTANOIC ACID, 3-METHYL-, (2R,3S,6S,7R,8R)-3-((3-(FORMYLAMINO)-2-HYDROXYBENZOYL)AMINO)-8-HEXYL-2,6-DIMETHYL-4,9-DIOXO-1,5-DIOXONAN-7-YL ESTER; isovaleric acid 8-ester with 3-formamido-N-(7-hexyl-8-hydroxy-4,9-dimethyl-2,6-dioxo-1,5-dioxonan-3-yl)salicylamide
|
|
| CAS | 1397-94-0 | |
| PubChem CID | 14957 | |
| ChEMBL ID | CHEMBL211501 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 548.6 | ALogp: | 5.3 |
| HBD: | 3 | HBA: | 9 |
| Rotatable Bonds: | 12 | Lipinski's rule of five: | Rejected |
| Polar Surface Area: | 157.0 | Aromatic Rings: | 2 |
| Heavy Atoms: | 39 | QED Weighted: | 0.114 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.748 | MDCK Permeability: | 0.00004200 |
| Pgp-inhibitor: | 0.73 | Pgp-substrate: | 0.006 |
| Human Intestinal Absorption (HIA): | 0.005 | 20% Bioavailability (F20%): | 0.009 |
| 30% Bioavailability (F30%): | 0.983 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.878 | Plasma Protein Binding (PPB): | 89.27% |
| Volume Distribution (VD): | 0.614 | Fu: | 6.64% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.142 | CYP1A2-substrate: | 0.08 |
| CYP2C19-inhibitor: | 0.56 | CYP2C19-substrate: | 0.094 |
| CYP2C9-inhibitor: | 0.82 | CYP2C9-substrate: | 0.957 |
| CYP2D6-inhibitor: | 0.18 | CYP2D6-substrate: | 0.135 |
| CYP3A4-inhibitor: | 0.911 | CYP3A4-substrate: | 0.143 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 2.063 | Half-life (T1/2): | 0.343 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.012 | Human Hepatotoxicity (H-HT): | 0.792 |
| Drug-inuced Liver Injury (DILI): | 0.896 | AMES Toxicity: | 0.012 |
| Rat Oral Acute Toxicity: | 0.029 | Maximum Recommended Daily Dose: | 0.013 |
| Skin Sensitization: | 0.105 | Carcinogencity: | 0.183 |
| Eye Corrosion: | 0.003 | Eye Irritation: | 0.016 |
| Respiratory Toxicity: | 0.016 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
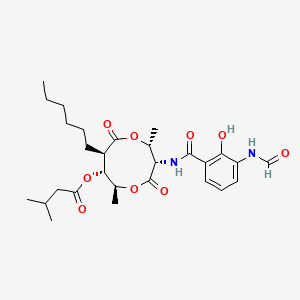
| ENC001023 | 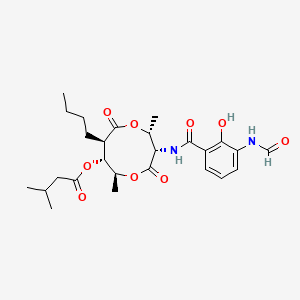 |
0.925 | D0T9TJ |  |
0.275 | ||
| ENC001502 |  |
0.858 | D07IPB |  |
0.251 | ||
| ENC001503 | 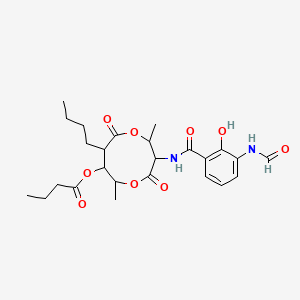 |
0.805 | D00OAY | 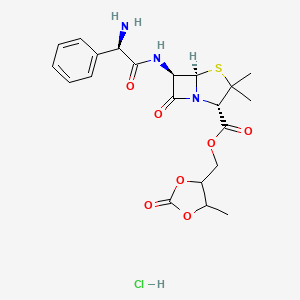 |
0.250 | ||
| ENC004061 | 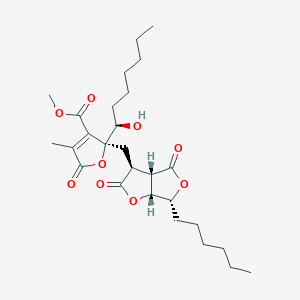 |
0.306 | D0T5XN | 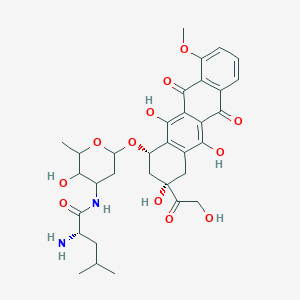 |
0.246 | ||
| ENC001801 |  |
0.281 | D0K8CI |  |
0.234 | ||
| ENC000878 |  |
0.276 | D06TQZ |  |
0.231 | ||
| ENC003631 |  |
0.272 | D0HD9K |  |
0.230 | ||
| ENC002378 |  |
0.269 | D04VEJ |  |
0.228 | ||
| ENC002514 | 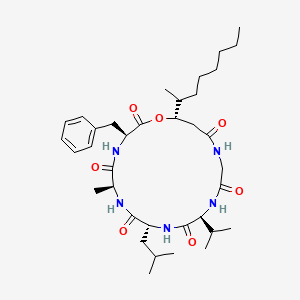 |
0.267 | D0H2YX | 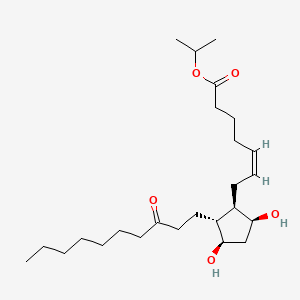 |
0.227 | ||
| ENC003015 | 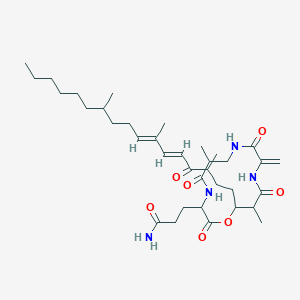 |
0.266 | D0ZI4H | 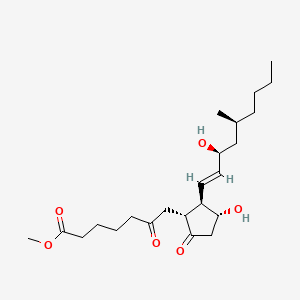 |
0.225 | ||