NPs Basic Information
|
Name |
Isosorbide
|
| Molecular Formula | C6H10O4 | |
| IUPAC Name* |
(3S,3aR,6R,6aR)-2,3,3a,5,6,6a-hexahydrofuro[3,2-b]furan-3,6-diol
|
|
| SMILES |
C1[C@H]([C@@H]2[C@H](O1)[C@H](CO2)O)O
|
|
| InChI |
InChI=1S/C6H10O4/c7-3-1-9-6-4(8)2-10-5(3)6/h3-8H,1-2H2/t3-,4+,5-,6-/m1/s1
|
|
| InChIKey |
KLDXJTOLSGUMSJ-JGWLITMVSA-N
|
|
| Synonyms |
isosorbide; 652-67-5; Isobide; Devicoran; Hydronol; Ismotic; 1,4:3,6-Dianhydro-D-glucitol; Sorbid; (3R,3aR,6S,6aR)-hexahydrofuro[3,2-b]furan-3,6-diol; (+)-D-Isosorbide; Vascardin dinitrate; Dianhydro-D-glucitol; D-Glucitol, 1,4:3,6-dianhydro-; 1,4-Dianhydrosorbitol; AT-101; 1,4:3,6-Dianhydro-D-sorbitol; 1,4:3,6-Dianhydrosorbitol; D-Isosorbide; (3S,3aR,6R,6aR)-2,3,3a,5,6,6a-hexahydrofuro[3,2-b]furan-3,6-diol; NSC-40725; WXR179L51S; Sorbitol, 1,4:3,6-dianhydro-; NSC40725; D-1,4:3,6-Dianhydroglucitol; NCGC00160508-01; Isosorbide 100 microg/mL in Acetonitrile; Glucitol, 1,4:3,6-dianhydro-, D-; DSSTox_CID_26196; DSSTox_RID_81427; DSSTox_GSID_46196; Hydronol (VAN); Isosorbida; Isosorbidum; Isosorbidum [INN-Latin]; Isosorbida [INN-Spanish]; CAS-652-67-5; HSDB 3105; EINECS 211-492-3; NSC 40725; BRN 0080510; UNII-WXR179L51S; Ismotic (TN); Isobide (TN); MFCD00064827; Isosorbide [USAN:USP:INN:BAN:JAN]; 1,6-Dianhydrosorbitol; ISOSORBIDE [MI]; ISOSORBIDE [INN]; ISOSORBIDE [JAN]; ISOSORBIDE [HSDB]; ISOSORBIDE [INCI]; ISOSORBIDE [USAN]; ISOSORBIDE [VANDF]; 1,6-Dianhydro-D-glucitol; 1,6-Dianhydro-D-sorbitol; EC 211-492-3; ISOSORBIDE [MART.]; ISOSORBIDE [USP-RS]; ISOSORBIDE [WHO-DD]; Dianhydro-D-glucitol, 98%; SCHEMBL15495; 1,4:3,6-Dianhydroglucitol; 5-19-03-00201 (Beilstein Handbook Reference); BIDD:GT0695; 1,4; 3,6-dianhydrosorbitol; CHEBI:6060; Isosorbide (JP17/USP/INN); D-Glucitol,4:3,6-dianhydro-; CHEMBL1200660; DTXSID5046196; ISOSORBIDE [ORANGE BOOK]; 1.4:3.6-dianhydro-D-glucitol; 1.4;3.6-dianhydro-D-glucitol; ISOSORBIDE [USP IMPURITY]; Glucitol,4:3,6-dianhydro-, D-; HY-B1469; Tox21_111861; BBL029591; s4204; STK801813; ZINC18284778; AKOS005622709; Tox21_111861_1; CCG-266173; CS-5157; DB09401; SMP1_000177; NCGC00160508-02; NCGC00160508-03; AS-14140; I0407; D00347; EN300-170910; AB01566931_01; Q1243800; Z1216815730; A912284D-27E1-4FB0-91B8-86C8AB905297; WURCS=2.0/1,1,0/[h2122h_1-4_3-6]/1/; D-Sorbitol, {1,4:3,6-dianhydro(furo[3,2-b]furan-3,6-diol,} hexahydro-); D-Sorbitol,4:3,6-dianhydro(furo[3,2-b]furan-3,6-diol, hexahydro-)
|
|
| CAS | 652-67-5 | |
| PubChem CID | 12597 | |
| ChEMBL ID | CHEMBL1200660 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 146.14 | ALogp: | -1.4 |
| HBD: | 2 | HBA: | 4 |
| Rotatable Bonds: | 0 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 58.9 | Aromatic Rings: | 2 |
| Heavy Atoms: | 10 | QED Weighted: | 0.462 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -5.09 | MDCK Permeability: | 0.00007460 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.969 |
| Human Intestinal Absorption (HIA): | 0.011 | 20% Bioavailability (F20%): | 0.188 |
| 30% Bioavailability (F30%): | 0.276 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.393 | Plasma Protein Binding (PPB): | 13.20% |
| Volume Distribution (VD): | 1.388 | Fu: | 85.80% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.03 | CYP1A2-substrate: | 0.103 |
| CYP2C19-inhibitor: | 0.031 | CYP2C19-substrate: | 0.416 |
| CYP2C9-inhibitor: | 0.007 | CYP2C9-substrate: | 0.058 |
| CYP2D6-inhibitor: | 0.009 | CYP2D6-substrate: | 0.238 |
| CYP3A4-inhibitor: | 0.011 | CYP3A4-substrate: | 0.07 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 2.413 | Half-life (T1/2): | 0.663 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.102 | Human Hepatotoxicity (H-HT): | 0.689 |
| Drug-inuced Liver Injury (DILI): | 0.09 | AMES Toxicity: | 0.136 |
| Rat Oral Acute Toxicity: | 0.652 | Maximum Recommended Daily Dose: | 0.058 |
| Skin Sensitization: | 0.658 | Carcinogencity: | 0.183 |
| Eye Corrosion: | 0.521 | Eye Irritation: | 0.834 |
| Respiratory Toxicity: | 0.778 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
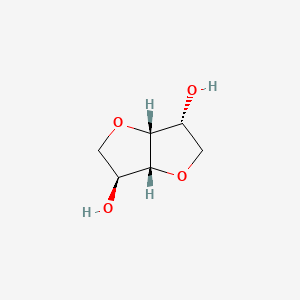
| ENC003056 | 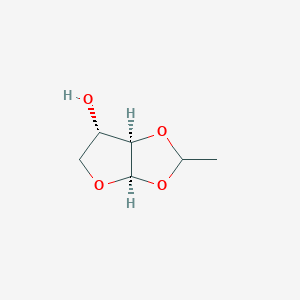 |
0.366 | D0YS7D | 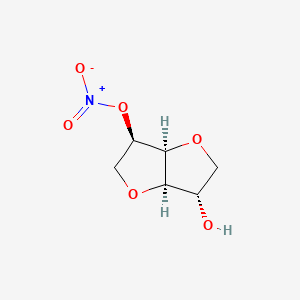 |
0.524 | ||
| ENC001251 | 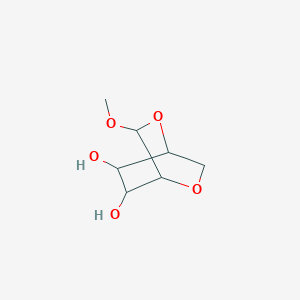 |
0.326 | D07HZY | 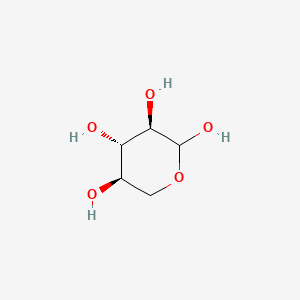 |
0.350 | ||
| ENC001252 | 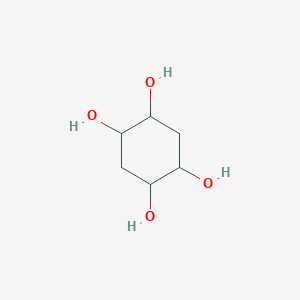 |
0.286 | D0I1SK | 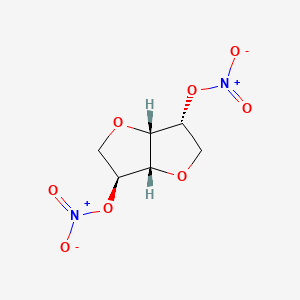 |
0.286 | ||
| ENC000767 | 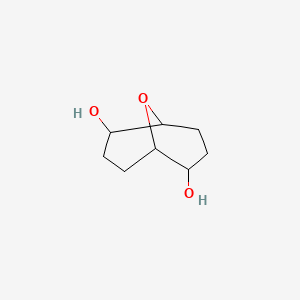 |
0.283 | D0Z4EI | 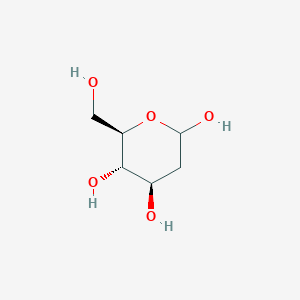 |
0.239 | ||
| ENC001003 | 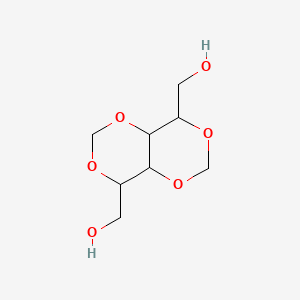 |
0.259 | D0MU9L | 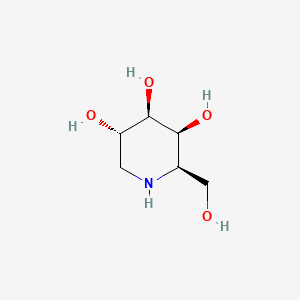 |
0.213 | ||
| ENC003037 | 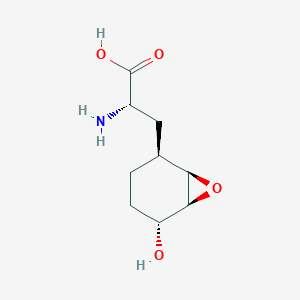 |
0.245 | D0D0ZD | 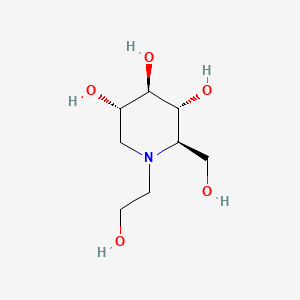 |
0.182 | ||
| ENC000927 | 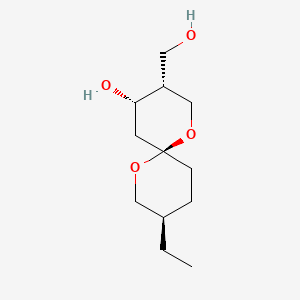 |
0.217 | D0H2RI | 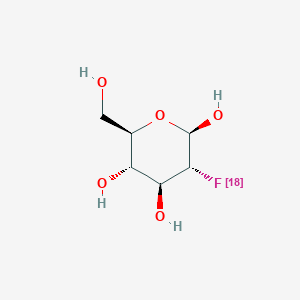 |
0.180 | ||
| ENC000928 | 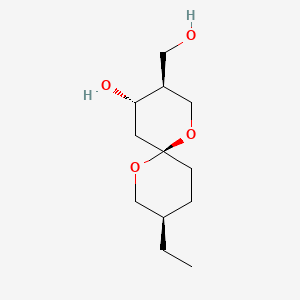 |
0.217 | D0H3KI | 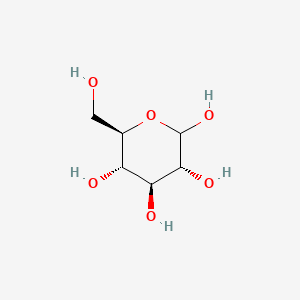 |
0.180 | ||
| ENC003431 | 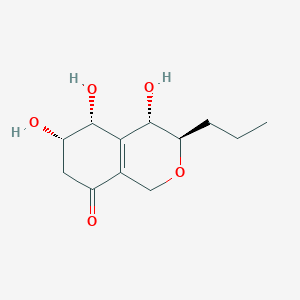 |
0.213 | D07NSU | 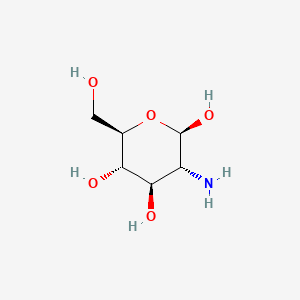 |
0.180 | ||
| ENC005293 | 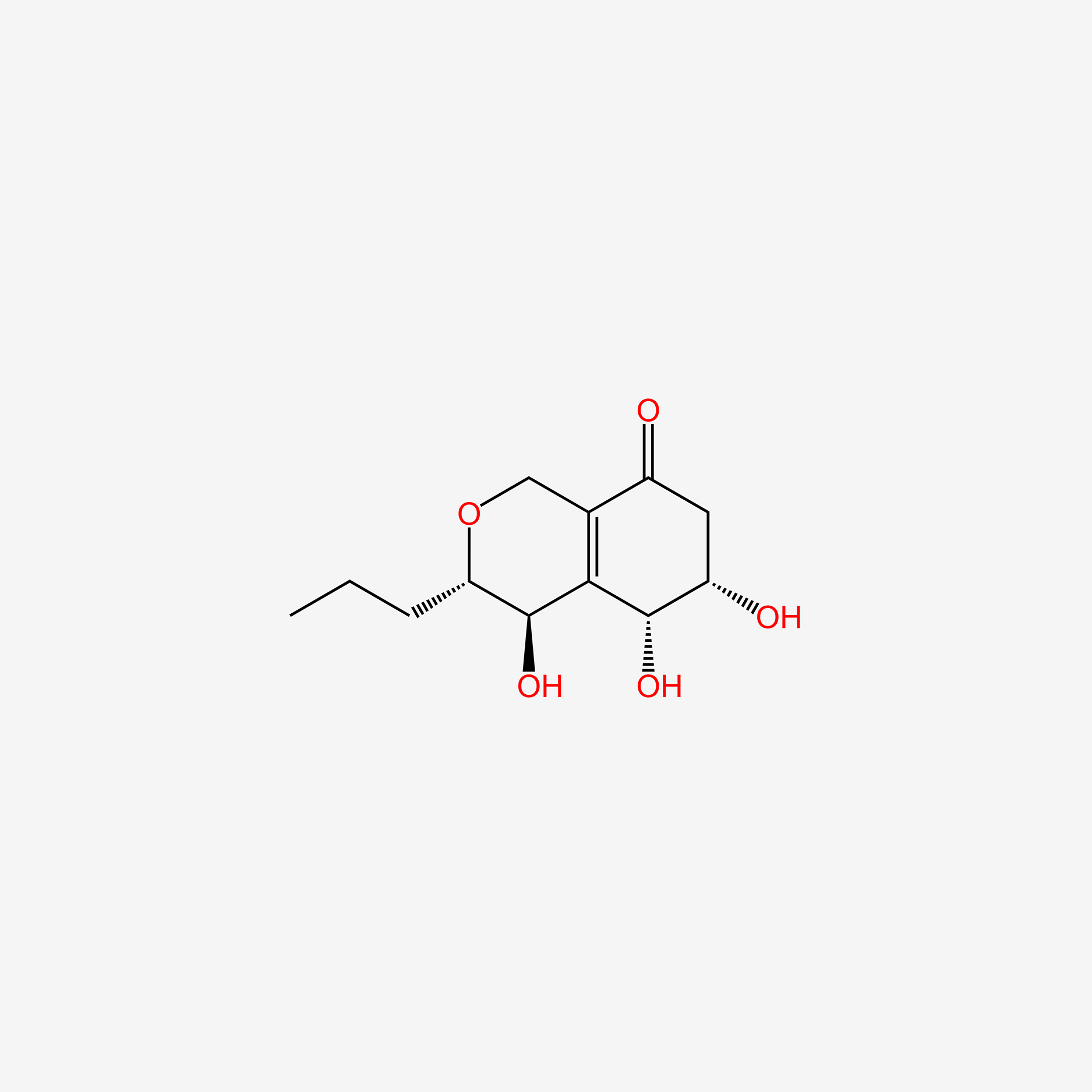 |
0.213 | D0F8CM |  |
0.178 | ||