NPs Basic Information
|
Name |
Isobutyl isobutyrate
|
| Molecular Formula | C8H16O2 | |
| IUPAC Name* |
2-methylpropyl 2-methylpropanoate
|
|
| SMILES |
CC(C)COC(=O)C(C)C
|
|
| InChI |
InChI=1S/C8H16O2/c1-6(2)5-10-8(9)7(3)4/h6-7H,5H2,1-4H3
|
|
| InChIKey |
RXGUIWHIADMCFC-UHFFFAOYSA-N
|
|
| Synonyms |
Isobutyl isobutyrate; 97-85-8; 2-Methylpropyl 2-methylpropanoate; Isobutyl isobutanoate; Isobutyl 2-methylpropanoate; 2-Methylpropyl 2-methylpropionate; Isobutyric acid, isobutyl ester; Propanoic acid, 2-methyl-, 2-methylpropyl ester; 2-METHYLPROPYL ISOBUTYRATE; Isobutylester kyseliny isomaselne; FEMA No. 2189; NSC 6538; 2-Methyl-1-propyl 2-methylpropanoate; Y495J99R5D; NSC-6538; Isobutyl ester of 2-methylpropanoic acid; WE(3:0(2Me)/3:0(2Me)); FEMA Number 2189; Isobutyl isobutyrate (natural); HSDB 5311; EINECS 202-612-5; UN2528; BRN 1701355; Isobutylester kyseliny isomaselne [Czech]; UNII-Y495J99R5D; AI3-06122; iso-butyl iso-butyrate; Isobutyric acid isobutyl; 2-methylpropylisobutyrate; 2-Methylpropanoic acid 2-methylpropyl ester; DSSTox_CID_6612; DSSTox_RID_78163; DSSTox_GSID_26612; SCHEMBL41548; 4-02-00-00847 (Beilstein Handbook Reference); Isobutyl isobutyrate [UN2528] [Flammable liquid]; Isobutyric Acid Isobutyl Ester; Isobutyl isobutyrate, >=97%; Isobutyl isobutyrate, >=98%; CHEMBL3188433; DTXSID6026612; FEMA 2189; ISOBUTYL ISOBUTYRATE [MI]; NSC6538; CHEBI:179934; Isobutyl Isobutyrate Reagent Grade; ZINC391110; ISOBUTYL ISOBUTYRATE [FHFI]; Tox21_201573; LMFA07010566; MFCD00008916; ISOBUTYL ISOBUTYRATE [USP-RS]; AKOS015837515; Isobutyl isobutyrate, natural, >=97%; UN 2528; CAS-97-85-8; NCGC00249072-01; NCGC00259122-01; 2-METHYLPROPYL ISOBUTYRATE [HSDB]; WLN: 1Y1 & VO1Y1 & 1; 2-METHYLPROPIONIC ACID ISOBUTYL ESTER; FT-0627346; I0316; Isobutyl isobutyrate [UN2528] [Flammable liquid]; Q26841351; Isobutyl isobutyrate, United States Pharmacopeia (USP) Reference Standard
|
|
| CAS | 97-85-8 | |
| PubChem CID | 7351 | |
| ChEMBL ID | CHEMBL3188433 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 144.21 | ALogp: | 2.5 |
| HBD: | 0 | HBA: | 2 |
| Rotatable Bonds: | 4 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 26.3 | Aromatic Rings: | 0 |
| Heavy Atoms: | 10 | QED Weighted: | 0.569 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.198 | MDCK Permeability: | 0.00003350 |
| Pgp-inhibitor: | 0.001 | Pgp-substrate: | 0.001 |
| Human Intestinal Absorption (HIA): | 0.009 | 20% Bioavailability (F20%): | 0.553 |
| 30% Bioavailability (F30%): | 0.577 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.978 | Plasma Protein Binding (PPB): | 49.37% |
| Volume Distribution (VD): | 1.077 | Fu: | 64.24% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.647 | CYP1A2-substrate: | 0.193 |
| CYP2C19-inhibitor: | 0.11 | CYP2C19-substrate: | 0.9 |
| CYP2C9-inhibitor: | 0.134 | CYP2C9-substrate: | 0.465 |
| CYP2D6-inhibitor: | 0.008 | CYP2D6-substrate: | 0.156 |
| CYP3A4-inhibitor: | 0.014 | CYP3A4-substrate: | 0.31 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 8.741 | Half-life (T1/2): | 0.807 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.014 | Human Hepatotoxicity (H-HT): | 0.021 |
| Drug-inuced Liver Injury (DILI): | 0.419 | AMES Toxicity: | 0.023 |
| Rat Oral Acute Toxicity: | 0.065 | Maximum Recommended Daily Dose: | 0.014 |
| Skin Sensitization: | 0.238 | Carcinogencity: | 0.117 |
| Eye Corrosion: | 0.949 | Eye Irritation: | 0.98 |
| Respiratory Toxicity: | 0.269 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000819 |  |
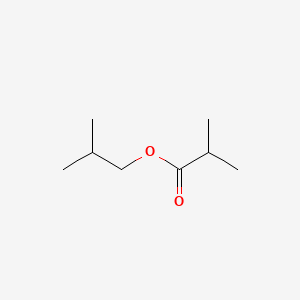
0.656 | D0B2OT | 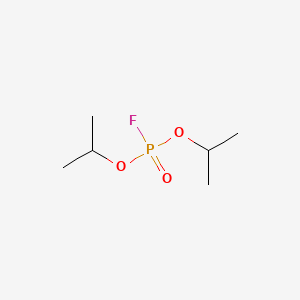 |
0.300 | ||
| ENC001137 | 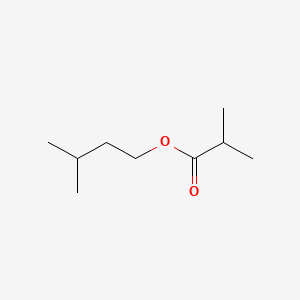 |
0.656 | D0ZK8H |  |
0.286 | ||
| ENC000397 | 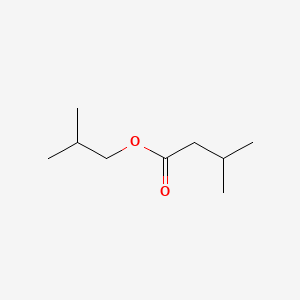 |
0.606 | D0U9QU | 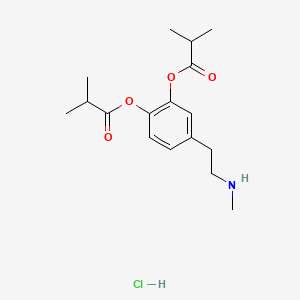 |
0.269 | ||
| ENC000246 | 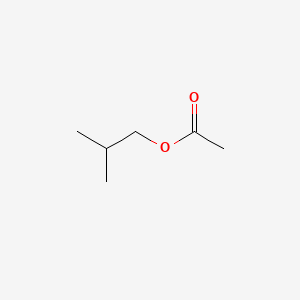 |
0.552 | D04MWJ | 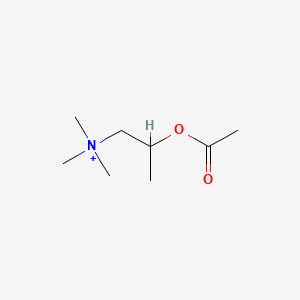 |
0.268 | ||
| ENC000186 | 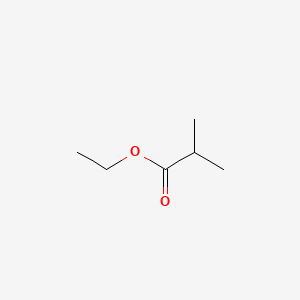 |
0.552 | D07ZTO | 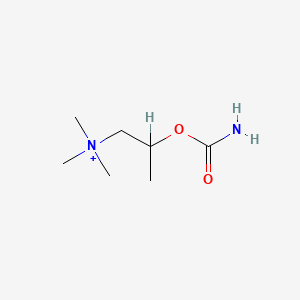 |
0.268 | ||
| ENC000188 | 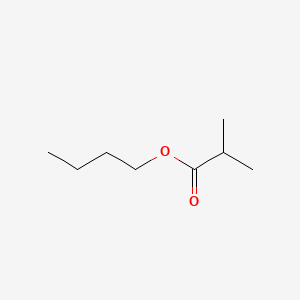 |
0.457 | D00WUF | 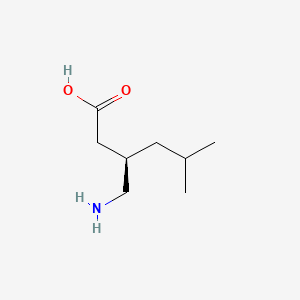 |
0.262 | ||
| ENC000382 | 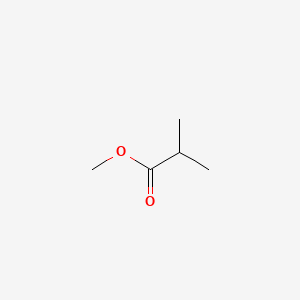 |
0.448 | D0Q9HF | 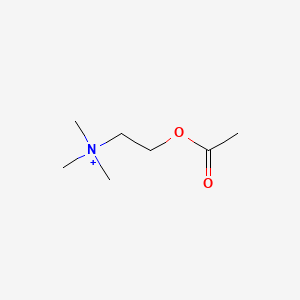 |
0.250 | ||
| ENC000448 | 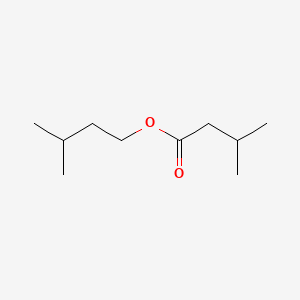 |
0.436 | D0R1QE | 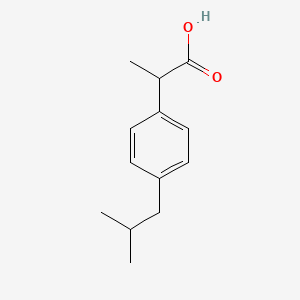 |
0.250 | ||
| ENC000771 |  |
0.424 | D03QJL |  |
0.246 | ||
| ENC000726 |  |
0.421 | D0P7VJ |  |
0.226 | ||