NPs Basic Information
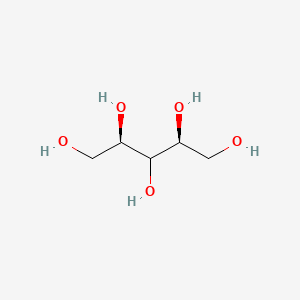
|
Name |
Xylitol
|
| Molecular Formula | C5H12O5 | |
| IUPAC Name* |
(2S,4R)-pentane-1,2,3,4,5-pentol
|
|
| SMILES |
C([C@H](C([C@H](CO)O)O)O)O
|
|
| InChI |
InChI=1S/C5H12O5/c6-1-3(8)5(10)4(9)2-7/h3-10H,1-2H2/t3-,4+,5?
|
|
| InChIKey |
HEBKCHPVOIAQTA-NGQZWQHPSA-N
|
|
| Synonyms |
xylitol; ribitol; adonitol; Xylite; 488-81-3; 87-99-0; D-Xylitol; Adonite; Adonit; D-ribitol; Xyliton; Eutrit; Klinit; Xylite (sugar); Kannit; Xylit; Newtol; 1,2,3,4,5-pentanepentol; Pentitol; Fluorette; Xylisorb; Kylit; meso-ribitol; xylo-Pentitol; (2R,3s,4S)-pentane-1,2,3,4,5-pentol; L-ribitol; (2R,3R,4S)-Pentane-1,2,3,4,5-pentaol; Xylitab 300; meso-xylitol; L-xylitol; D-Adonitol; (2S,4R)-pentane-1,2,3,4,5-pentol; NSC 25283; Xylitol, d-; Xylitol cm 90; BRN 1720523; 16277-71-7; CHEBI:15963; CHEBI:17151; xylo-Pentane-1,2,3,4,5-pentol; 2,3-Dihydro Acrivastine; C-xylidex cr 16055; 353ZQ9TVDA; VCQ006KQ1E; INS NO.967; (2R,3r,4S)-pentane-1,2,3,4,5-pentol; (2R,3S,4S)-Pentane-1,2,3,4,5-pentaol; Xylooligosaccharide; INS-967; NSC-25283; 87849-01-2; 1,2,3,4,5-Pentahydroxypentane; E-967; 4-01-00-02832 (Beilstein Handbook Reference); MFCD00064291; EINECS 201-788-0; UNII-353ZQ9TVDA; UNII-VCQ006KQ1E; Adonito; Xylitol [INN:BAN:JAN:NF]; NSC-16868; DL-Arabinit; Xylitol C; HSDB 7967; Xylitab DC; Wood sugar alcohol; RB0; Xylitol,(S); EINECS 207-685-7; Ribitol (Adonitol); Adonitol (7CI); Xylisorb 300; Xylisorb 700; MFCD00064292; NSC 16868; Xylitab 100; RIBO-PENTITOL; BRN 1720524; D-ribitol (incorrect); L-ribitol (incorrect); Adonitol, >=99%; XYLITOL [VANDF]; XYLITOL [INCI]; Xylitol, >=99%; ADONITOL [MI]; XYLITOL [FCC]; XYLITOL [JAN]; XYLITOL [II]; XYLITOL [MI]; XYLITOL [MART.]; XYLITOL [USP-RS]; XYLITOL [WHO-DD]; bmse000062; bmse000129; bmse000886; Epitope ID:114702; Epitope ID:114703; EC 201-788-0; SCHEMBL4250; Xylitol, analytical standard; DSSTox_CID_22514; DSSTox_RID_80046; DSSTox_GSID_42514; SCHEMBL15318; MLS002695898; CHEMBL96783; Ribitol (6CI,8CI,9CI); XYLITOL [EP IMPURITY]; XYLITOL [EP MONOGRAPH]; QSPL 191; SCHEMBL1924966; CHEMBL1865120; CHEMBL3137744; DTXSID601032335; HY-N0538; Tox21_201056; s2612; s4546; ZINC18068098; AKOS015903403; AKOS015915193; ZINC100014205; ZINC100018612; CCG-214167; CCG-266218; CS-6043; DB01904; DB11195; DB14704; CAS-87-99-0; NCGC00165982-01; NCGC00165982-02; NCGC00258609-01; NCGC00390798-01; Adonitol, BioXtra, >=99.0% (HPLC); AS-55964; DS-11416; E967; SMR001562099; HY-100582; Xylitol, Vetec(TM) reagent grade, >=99%; A0171; SW220290-1; X0018; Xylite 1000 microg/mL in Acetonitrile:Water; A-3000; Adonitol, BioReagent, suitable for cell culture; C00379; C00474; X-7000; EN300-7377714; EN300-7424092; WURCS=2.0/1,1,0/[h212h]/1/; WURCS=2.0/1,1,0/[h222h]/1/; A842433; Q212093; Q416534; Xylitol, European Pharmacopoeia (EP) Reference Standard; 5DCF4F57-E023-469A-B4F3-91E8349A6705; Xylitol, United States Pharmacopeia (USP) Reference Standard; 6684F574-C267-40CB-8828-12F2550E58D0; Xylitol, Pharmaceutical Secondary Standard; Certified Reference Material
|
|
| CAS | 87-99-0 | |
| PubChem CID | 6912 | |
| ChEMBL ID | CHEMBL96783 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 152.15 | ALogp: | -2.5 |
| HBD: | 5 | HBA: | 5 |
| Rotatable Bonds: | 4 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 101.0 | Aromatic Rings: | 0 |
| Heavy Atoms: | 10 | QED Weighted: | 0.309 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -5.249 | MDCK Permeability: | 0.00325770 |
| Pgp-inhibitor: | 0.004 | Pgp-substrate: | 0.039 |
| Human Intestinal Absorption (HIA): | 0.53 | 20% Bioavailability (F20%): | 0.241 |
| 30% Bioavailability (F30%): | 0.969 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.291 | Plasma Protein Binding (PPB): | 10.67% |
| Volume Distribution (VD): | 0.411 | Fu: | 82.46% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.016 | CYP1A2-substrate: | 0.035 |
| CYP2C19-inhibitor: | 0.01 | CYP2C19-substrate: | 0.068 |
| CYP2C9-inhibitor: | 0.001 | CYP2C9-substrate: | 0.091 |
| CYP2D6-inhibitor: | 0.001 | CYP2D6-substrate: | 0.117 |
| CYP3A4-inhibitor: | 0.004 | CYP3A4-substrate: | 0.017 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 1.757 | Half-life (T1/2): | 0.752 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.086 | Human Hepatotoxicity (H-HT): | 0.04 |
| Drug-inuced Liver Injury (DILI): | 0.033 | AMES Toxicity: | 0.054 |
| Rat Oral Acute Toxicity: | 0.005 | Maximum Recommended Daily Dose: | 0.002 |
| Skin Sensitization: | 0.064 | Carcinogencity: | 0.014 |
| Eye Corrosion: | 0.004 | Eye Irritation: | 0.682 |
| Respiratory Toxicity: | 0.016 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000405 | 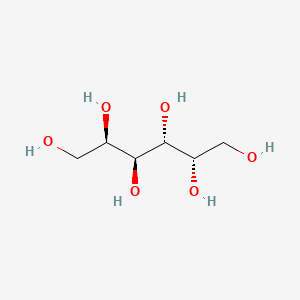 |
0.774 | D09MXS | 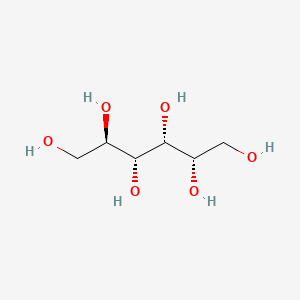 |
0.774 | ||
| ENC000136 | 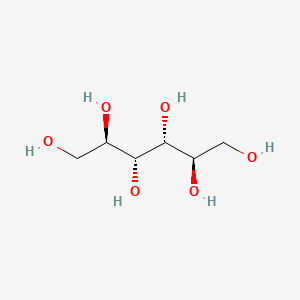 |
0.774 | D0P7EK | 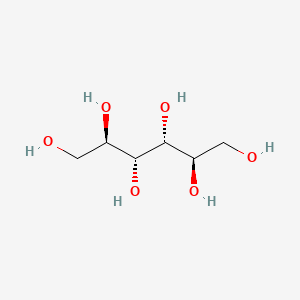 |
0.774 | ||
| ENC001758 | 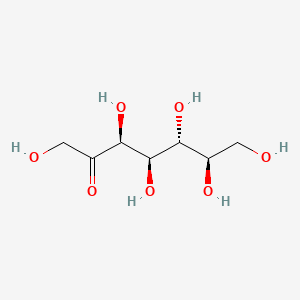 |
0.500 | D06HZY | 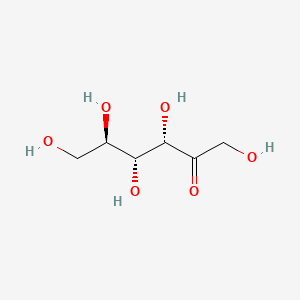 |
0.571 | ||
| ENC001267 | 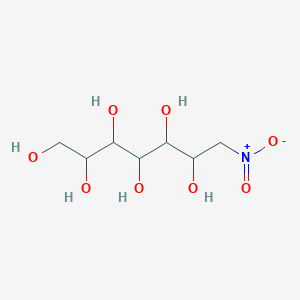 |
0.444 | D0VM8K | 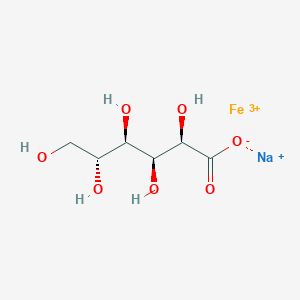 |
0.439 | ||
| ENC003038 | 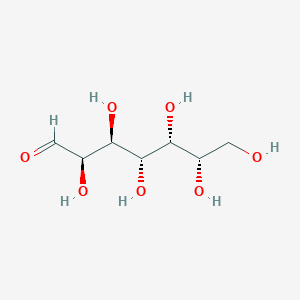 |
0.429 | D0T6VD | 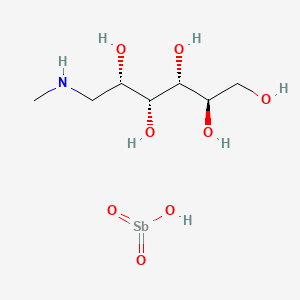 |
0.435 | ||
| ENC002398 | 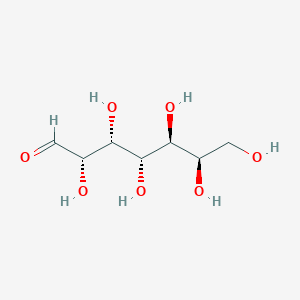 |
0.429 | D02KFP | 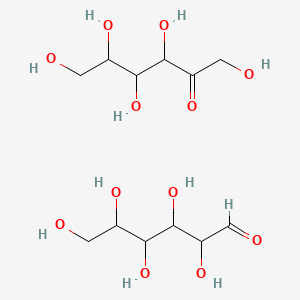 |
0.393 | ||
| ENC001002 | 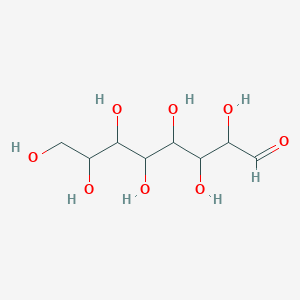 |
0.383 | D0B8SY | 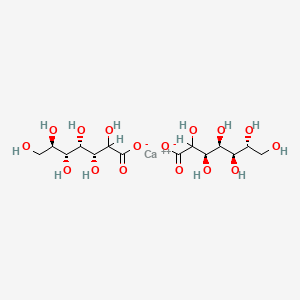 |
0.316 | ||
| ENC005325 | 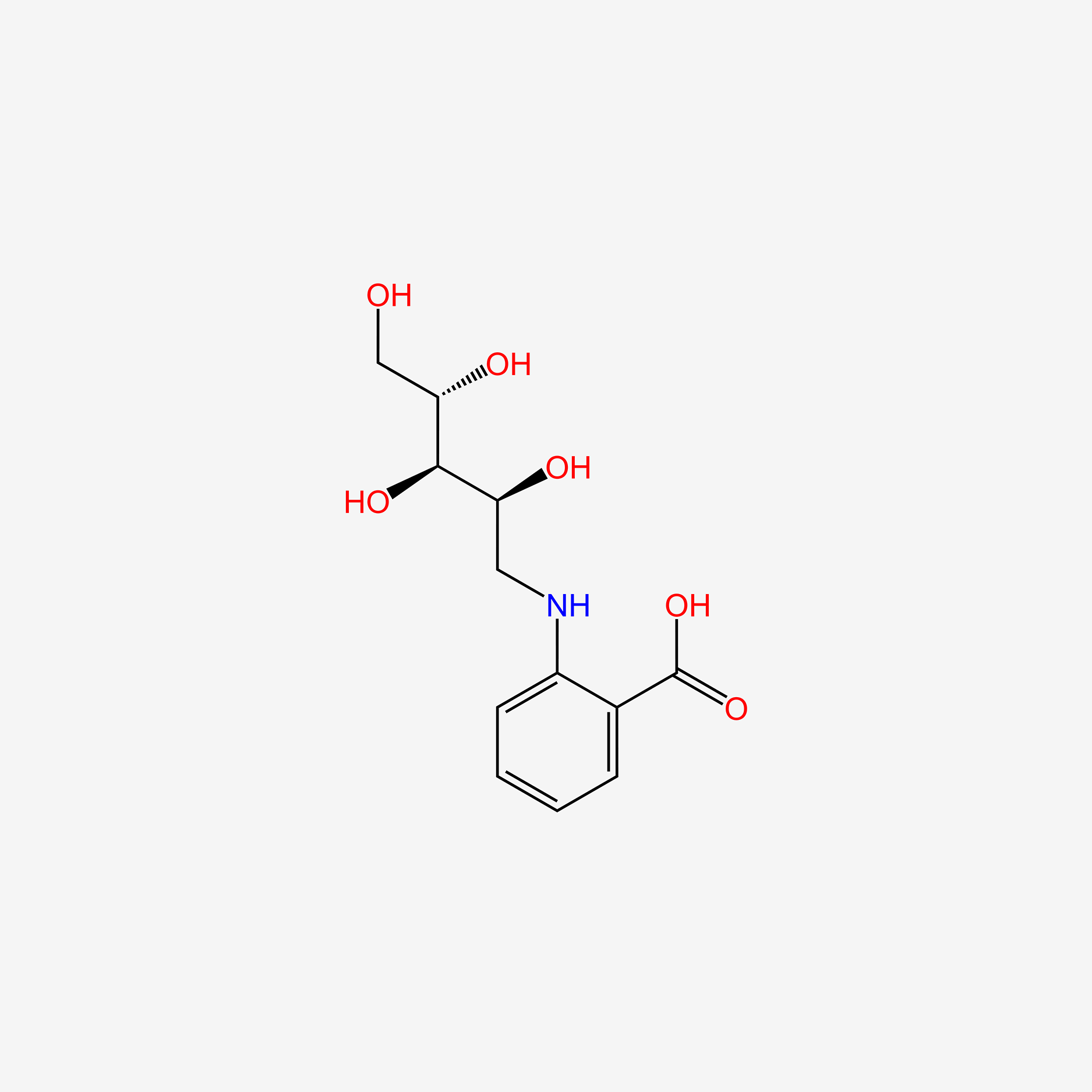 |
0.382 | D04QST | 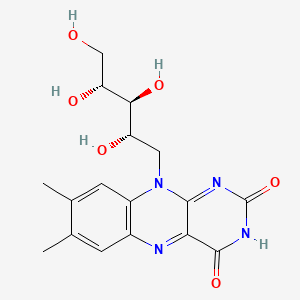 |
0.256 | ||
| ENC000040 | 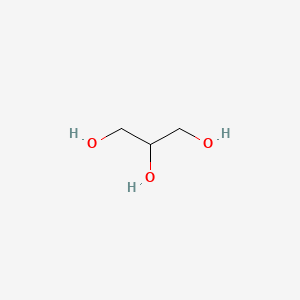 |
0.379 | D03MGL | 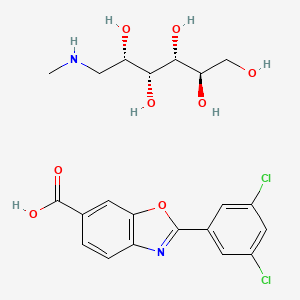 |
0.213 | ||
| ENC005982 | 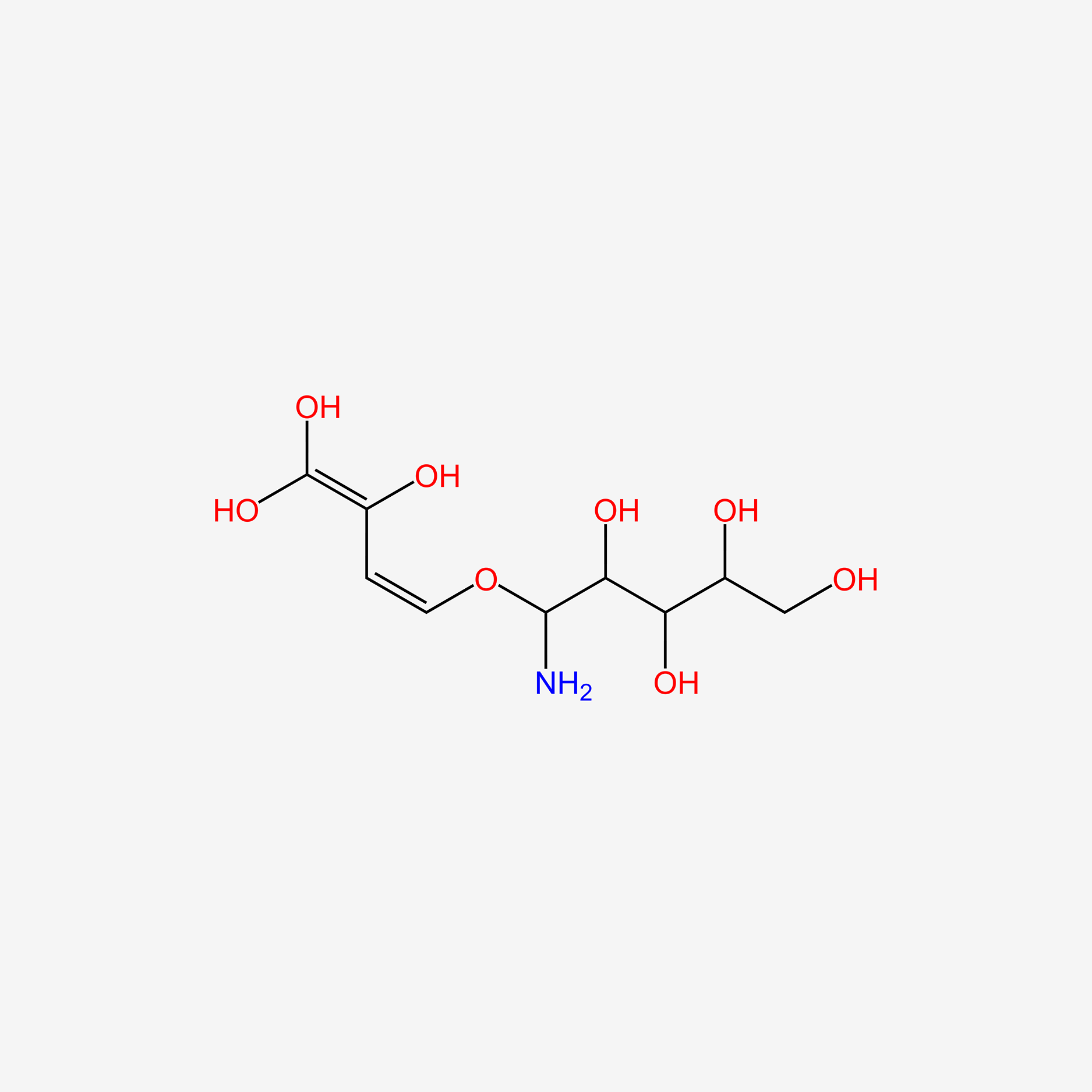 |
0.340 | D04ZTY | 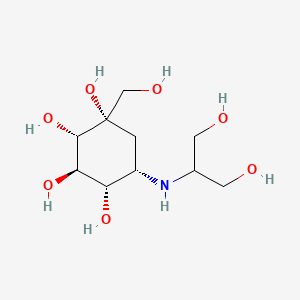 |
0.200 | ||