NPs Basic Information
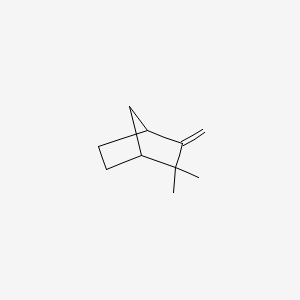
|
Name |
Camphene
|
| Molecular Formula | C10H16 | |
| IUPAC Name* |
2,2-dimethyl-3-methylidenebicyclo[2.2.1]heptane
|
|
| SMILES |
CC1(C2CCC(C2)C1=C)C
|
|
| InChI |
InChI=1S/C10H16/c1-7-8-4-5-9(6-8)10(7,2)3/h8-9H,1,4-6H2,2-3H3
|
|
| InChIKey |
CRPUJAZIXJMDBK-UHFFFAOYSA-N
|
|
| Synonyms |
CAMPHENE; 79-92-5; Comphene; 2,2-Dimethyl-3-methylenenorbornane; (+/-)-Camphene; 2,2-dimethyl-3-methylidenebicyclo[2.2.1]heptane; 3,3-Dimethyl-2-methylenenorbornane; 3,3-Dimethyl-2-methylenenorcamphane; 565-00-4; 2,2-Dimethyl-3-methylenebicyclo[2.2.1]heptane; Bicyclo[2.2.1]heptane, 2,2-dimethyl-3-methylene-; FEMA No. 2229; DL-Camphene; Bicyclo(2.2.1)heptane, 2,2-dimethyl-3-methylene-; CHEBI:3830; G3VG94Z26E; Camphene, (1R,4S)-(+)-; 2,2-Dimethyl-3-methylenebicyclo(2.2.1)heptane; NSC-4165; DSSTox_CID_6488; DSSTox_RID_78121; DSSTox_GSID_26488; Bicyclo[2.2.1]heptane, 2,2-dimethyl-3-methylene-, (1R)-; CAS-79-92-5; CCRIS 3783; HSDB 900; NSC 4165; 3,3-Dimethyl-2-methylenenorcamphene; EINECS 201-234-8; EINECS 209-275-3; UNII-G3VG94Z26E; AI3-01775; Camphene (2,2-dimethyl-3-methylene-norbornane); 2,2-dimethyl-3-methylene-norbornane; MFCD00066603; CAMPHENE [FHFI]; CAMPHENE [HSDB]; CAMPHENE [INCI]; CAMPHENE [FCC]; CAMPHENE [MI]; CAMPHENE, DL-; 2,2-Dimethyl-3-methylenebicyclo[2.2.1]heptane #; CAMPHENE, D,L-; CAMPHENE [MART.]; CAMPHENE [WHO-DD]; EC 201-234-8; 3,3-dimethyl-2-methylidenebicyclo[2.2.1]heptane; (1)-2,2-Dimethyl-3-methylenebicyclo(2.2.1)heptane; CAMPHENE, (+/-)-; CHEMBL2268550; DTXSID8026488; NSC4165; Tox21_202014; Tox21_303152; BBL033861; STK801857; AKOS004119935; CCG-266137; NCGC00249149-01; NCGC00257126-01; NCGC00259563-01; WLN: L55 A CYTJ CU1 D1 D1; VS-12317; DB-053130; DB-056393; DB-057848; FT-0609260; FT-0635856; FT-0635857; EN300-20391; C06076; E87135; Bicyclo[2.2.1]heptane,2-dimethyl-3-methylene-; 565C004; Q416775; SR-01000944833; SR-01000944833-1; (+/-)-Camphene, tech. (sum of camphene + fenchene); Z104478010
|
|
| CAS | 79-92-5 | |
| PubChem CID | 6616 | |
| ChEMBL ID | CHEMBL2268550 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 136.23 | ALogp: | 3.3 |
| HBD: | 0 | HBA: | 0 |
| Rotatable Bonds: | 0 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 0.0 | Aromatic Rings: | 2 |
| Heavy Atoms: | 10 | QED Weighted: | 0.444 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.463 | MDCK Permeability: | 0.00002040 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0 |
| Human Intestinal Absorption (HIA): | 0.003 | 20% Bioavailability (F20%): | 0.006 |
| 30% Bioavailability (F30%): | 0.003 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.975 | Plasma Protein Binding (PPB): | 67.76% |
| Volume Distribution (VD): | 1.761 | Fu: | 29.05% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.601 | CYP1A2-substrate: | 0.859 |
| CYP2C19-inhibitor: | 0.193 | CYP2C19-substrate: | 0.879 |
| CYP2C9-inhibitor: | 0.171 | CYP2C9-substrate: | 0.852 |
| CYP2D6-inhibitor: | 0.016 | CYP2D6-substrate: | 0.896 |
| CYP3A4-inhibitor: | 0.051 | CYP3A4-substrate: | 0.289 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 9.346 | Half-life (T1/2): | 0.077 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.015 | Human Hepatotoxicity (H-HT): | 0.143 |
| Drug-inuced Liver Injury (DILI): | 0.104 | AMES Toxicity: | 0.004 |
| Rat Oral Acute Toxicity: | 0.025 | Maximum Recommended Daily Dose: | 0.73 |
| Skin Sensitization: | 0.267 | Carcinogencity: | 0.194 |
| Eye Corrosion: | 0.913 | Eye Irritation: | 0.98 |
| Respiratory Toxicity: | 0.886 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000482 | 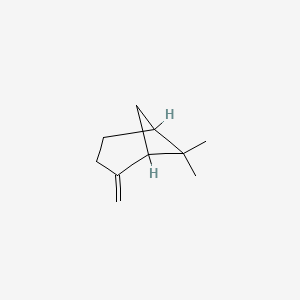 |
0.421 | D0V8HA | 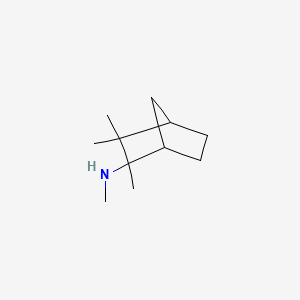 |
0.341 | ||
| ENC002998 | 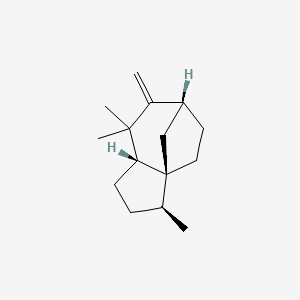 |
0.388 | D0H1QY |  |
0.273 | ||
| ENC001811 | 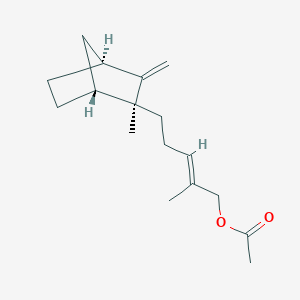 |
0.386 | D04CSZ | 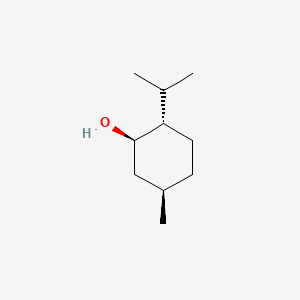 |
0.217 | ||
| ENC002228 | 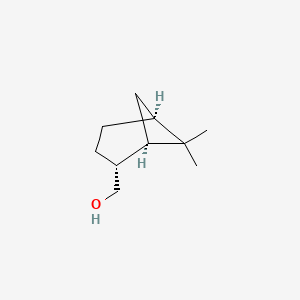 |
0.357 | D04DJN | 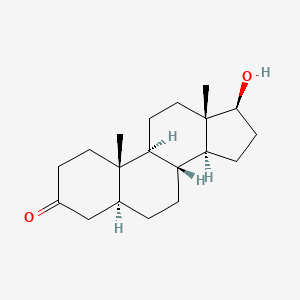 |
0.211 | ||
| ENC000613 | 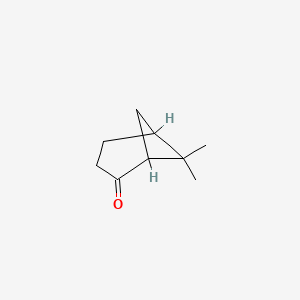 |
0.350 | D0U3GL | 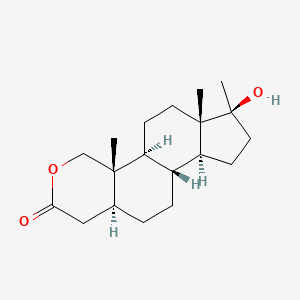 |
0.205 | ||
| ENC002084 |  |
0.333 | D0D2VS | 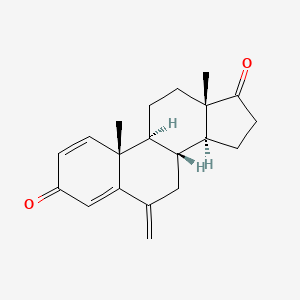 |
0.205 | ||
| ENC000366 | 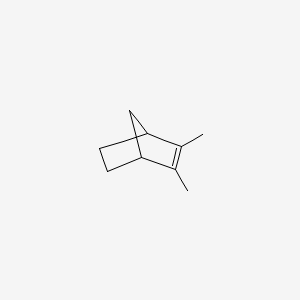 |
0.333 | D07QKN | 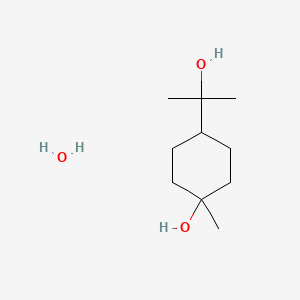 |
0.204 | ||
| ENC005520 | 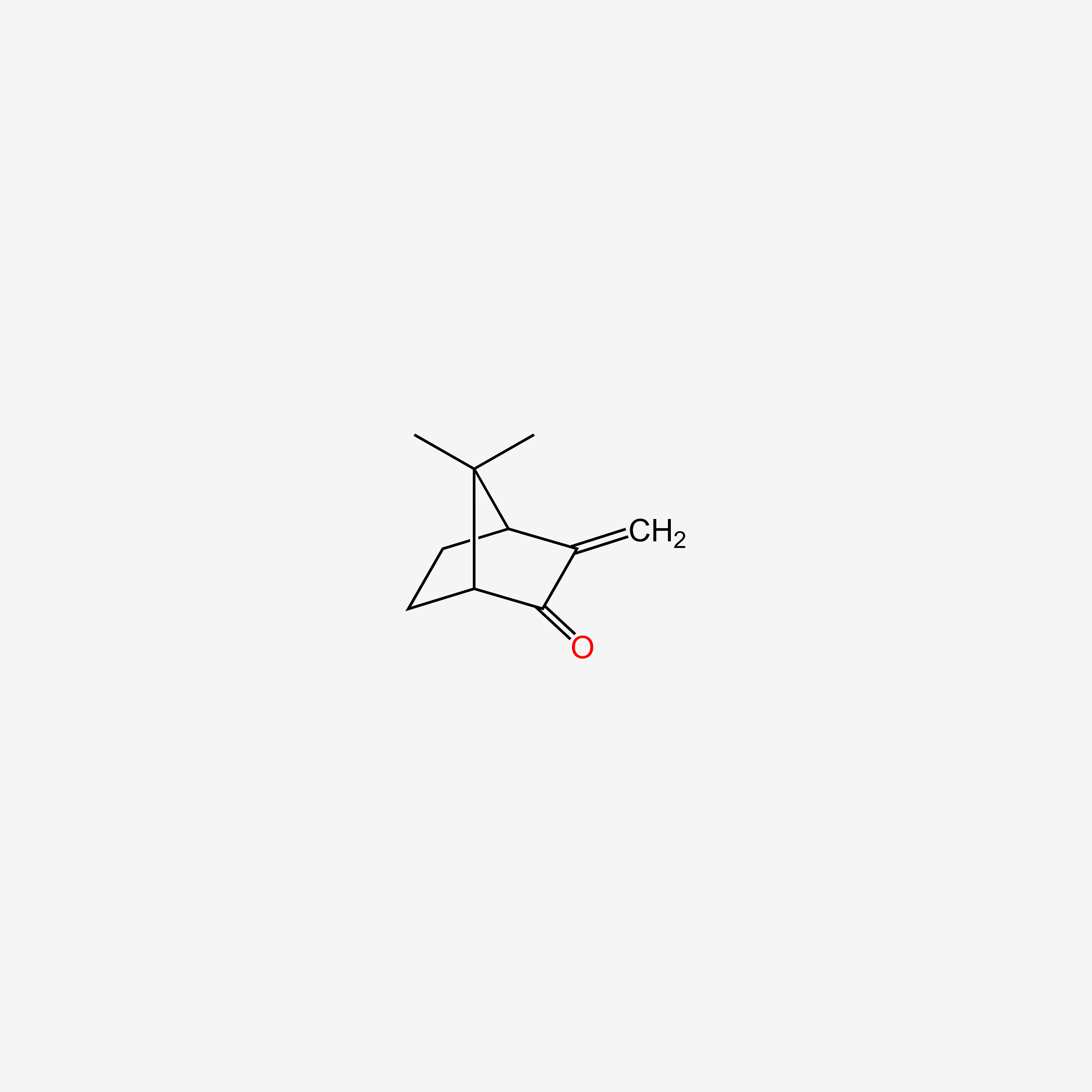 |
0.333 | D0A2AJ | 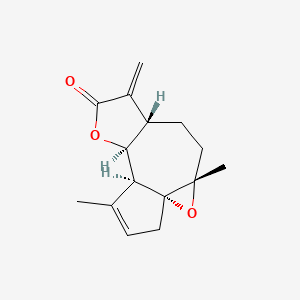 |
0.203 | ||
| ENC001814 | 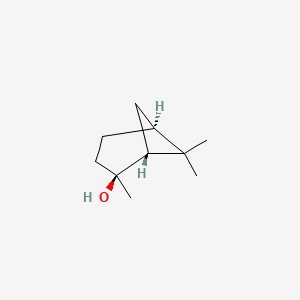 |
0.302 | D00VZZ |  |
0.197 | ||
| ENC000481 |  |
0.302 | D0Q6NZ |  |
0.192 | ||