NPs Basic Information
|
Name |
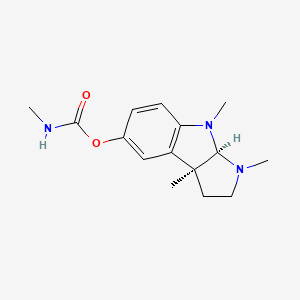
Physostigmine
|
| Molecular Formula | C15H21N3O2 | |
| IUPAC Name* |
[(3aR,8bS)-3,4,8b-trimethyl-2,3a-dihydro-1H-pyrrolo[2,3-b]indol-7-yl] N-methylcarbamate
|
|
| SMILES |
C[C@@]12CCN([C@@H]1N(C3=C2C=C(C=C3)OC(=O)NC)C)C
|
|
| InChI |
InChI=1S/C15H21N3O2/c1-15-7-8-17(3)13(15)18(4)12-6-5-10(9-11(12)15)20-14(19)16-2/h5-6,9,13H,7-8H2,1-4H3,(H,16,19)/t13-,15+/m1/s1
|
|
| InChIKey |
PIJVFDBKTWXHHD-HIFRSBDPSA-N
|
|
| Synonyms |
physostigmine; Eserine; 57-47-6; Antilirium; Physostol; Esromiotin; (-)-physostigmine; Ezerin; Calabarine; Erserine; Fysostigmin; cogmine; (3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydropyrrolo[2,3-b]indol-5-yl methylcarbamate; eserin; Eserinum; Eserolein, methylcarbamate (ester); Eserine sulfate; CHEMBL94; NSC-30782; 9U1VM840SP; CHEBI:27953; MCV-4484; NSC30782; NCGC00093889-03; DSSTox_CID_3471; [(3aR,8bS)-3,4,8b-trimethyl-2,3a-dihydro-1H-pyrrolo[2,3-b]indol-7-yl] N-methylcarbamate; DSSTox_RID_77040; DSSTox_GSID_23471; Pyrrolo(2,3-b)indol-5-ol, 1,2,3,3a,8,8a-hexahydro-1,3a,8-trimethyl-, methylcarbamate (ester), (3aS-cis); Fysostigmin [Czech]; CHEMBL537674; Pyrrolo(2,3-b)indol-5-ol, 1,2,3,3a,8,8a-hexahydro-1,3a,8-trimethyl-, methylcarbamate (ester), (3aS-cis)-; (3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyrrolo[2,3-b]indol-5-yl N-methylcarbamate; CAS-57-47-6; CCRIS 3422; HSDB 3161; MCV 4484; EINECS 200-332-8; Eserolein, methylcarbamate; NIH 10421; NSC 30782; Physostigmine [USP:BAN]; RCRA waste no. P204; physostigmin; UNII-9U1VM840SP; CS 58525; Methyl-carbamic acid, ester with eseroline; Carbamic acid, methyl-, ester with eseroline; (-) physostigmine; Eserine (TN); Physostigmine (USP); Spectrum_000916; Spectrum_001789; ESERINUM [HPUS]; SpecPlus_000381; Prestwick0_000566; Prestwick1_000566; Prestwick2_000566; Prestwick3_000566; Spectrum2_000330; Spectrum2_000757; Spectrum2_001283; Spectrum3_000545; Spectrum3_000901; Spectrum4_000997; Spectrum4_001631; Spectrum4_001913; Spectrum5_000441; Spectrum5_000626; Spectrum5_001672; PHYSOSTIGMINE [MI]; physostigmine.salicylic acid; 1,2,3,3abeta,8abeta-Hexahydro-1,3a,8-trimethylpyrrolo(2,3-b)-indol-5-yl methylcarbamate; PHYSOSTIGMINE [HSDB]; Lopac0_000483; SCHEMBL24044; BSPBio_000352; BSPBio_002189; KBioGR_001433; KBioGR_002061; KBioGR_002533; KBioSS_001396; KBioSS_002279; PHYSOSTIGMINE [VANDF]; (3aS-cis)-1,2,3,3a,8,8a-Hexahydro-1,3a,8-trimethylpyrrolo(2,3-b)indol-5-ol methylcarbamate (ester); MLS001304022; DivK1c_006477; PHYSOSTIGMINE [MART.]; SPECTRUM1500753; SPBio_000339; SPBio_000774; SPBio_001285; SPBio_002571; PHYSOSTIGMINE [WHO-DD]; BPBio1_000388; cid_657348; GTPL6598; MEGxp0_001872; DTXSID3023471; ACon1_000097; BDBM11023; KBio1_001421; KBio2_001396; KBio2_002278; KBio2_003964; KBio2_004846; KBio2_006532; KBio2_007414; KBio3_001689; KBio3_001842; Eserine, >=98.0% (N); HMS1921G06; HMS2089M11; HMS2236L08; HMS3261B07; BCP19735; Carbamic acid, ester with eseroline; HY-N6608; PHYSOSTIGMINE [USP IMPURITY]; Tox21_111228; Tox21_301591; Tox21_500483; BDBM50004000; BDBM50222010; CCG-38605; MFCD00151090; ZINC91689892; AKOS016843649; Tox21_111228_1; DB00981; LP00483; SDCCGMLS-0066585.P001; SDCCGSBI-0050467.P005; NCGC00093889-01; NCGC00093889-02; NCGC00093889-04; NCGC00093889-05; NCGC00093889-06; NCGC00093889-07; NCGC00093889-08; NCGC00093889-09; NCGC00093889-10; NCGC00093889-12; NCGC00093889-13; NCGC00093889-20; NCGC00255345-01; NCGC00261168-01; AC-15983; Pyrrolo(2,3-b)indol-5-ol, 1,2,3,3a,8,8a-hexahydro-1,3a,8-trimethyl-, methylcarbamate (ester), (3aS,8aR)-; SMR000718753; SBI-0050467.P004; CS-0034353; EU-0100483; P0406; Physostigmine 100 microg/mL in Acetonitrile; C06535; D00196; E 8375; EN300-23839407; Q410595; Eserine; Antilirium; Physostol; Esromiotin; Ezerin; SR-01000075341-1; WLN: T B556 EN GNTT&J B1 E1 G1 KOVM1; BRD-K25650355-001-02-5; BRD-K25650355-059-02-3; BRD-K25650355-059-12-2; (3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydropyrrolo[2,3-b]indol-5-yl methylcarbamate hydrochloride; (3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyrrolo[2,3-b]indol-5-yl N-methylcarbamate; 2-hydroxybenzoic acid; 1,2,3,3A.BETA.,8A.BETA.-HEXAHYDRO-1,3A,8-TRIMETHYLPYRROLO(2,3-B)-INDOL-5-YL METHYLCARBAMATE; Pyrrolo(2,3-b)indol-5-ol, 1,2,3,3a,8,8a-hexahydro-1,3a,8-trimethyl-, 5-(N-methylcarbamate), (3aS,8aR)-; Pyrrolo[2, 1,2,3,3a,8,8a-hexahydro-1,3a,8-trimethyl-, methylcarbamate (ester), (3aS-cis)-
|
|
| CAS | 57-47-6 | |
| PubChem CID | 5983 | |
| ChEMBL ID | CHEMBL94 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 275.35 | ALogp: | 0.7 |
| HBD: | 1 | HBA: | 4 |
| Rotatable Bonds: | 2 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 44.8 | Aromatic Rings: | 3 |
| Heavy Atoms: | 20 | QED Weighted: | 0.854 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.884 | MDCK Permeability: | 0.00002150 |
| Pgp-inhibitor: | 0.054 | Pgp-substrate: | 0.824 |
| Human Intestinal Absorption (HIA): | 0.981 | 20% Bioavailability (F20%): | 0.996 |
| 30% Bioavailability (F30%): | 0.998 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.993 | Plasma Protein Binding (PPB): | 37.48% |
| Volume Distribution (VD): | 1.456 | Fu: | 78.58% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.155 | CYP1A2-substrate: | 0.316 |
| CYP2C19-inhibitor: | 0.082 | CYP2C19-substrate: | 0.936 |
| CYP2C9-inhibitor: | 0.02 | CYP2C9-substrate: | 0.247 |
| CYP2D6-inhibitor: | 0.535 | CYP2D6-substrate: | 0.881 |
| CYP3A4-inhibitor: | 0.029 | CYP3A4-substrate: | 0.224 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 7.772 | Half-life (T1/2): | 0.607 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.143 | Human Hepatotoxicity (H-HT): | 0.031 |
| Drug-inuced Liver Injury (DILI): | 0.051 | AMES Toxicity: | 0.353 |
| Rat Oral Acute Toxicity: | 0.989 | Maximum Recommended Daily Dose: | 0.902 |
| Skin Sensitization: | 0.38 | Carcinogencity: | 0.876 |
| Eye Corrosion: | 0.003 | Eye Irritation: | 0.013 |
| Respiratory Toxicity: | 0.906 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000081 | 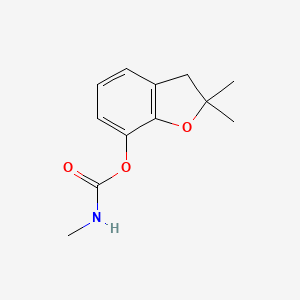 |
0.338 | D09OBB | 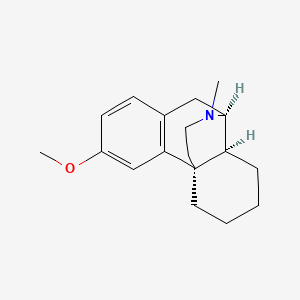 |
0.299 | ||
| ENC004190 | 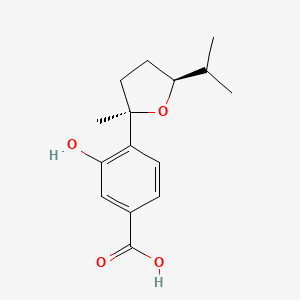 |
0.262 | D0W6DG | 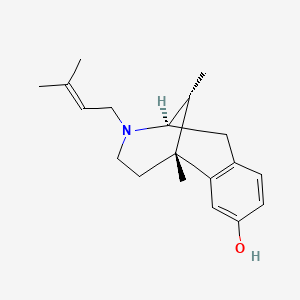 |
0.284 | ||
| ENC004191 | 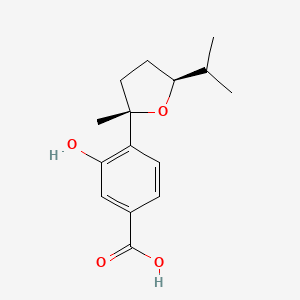 |
0.262 | D0WO8W | 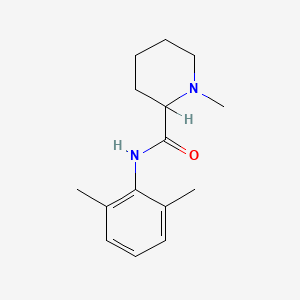 |
0.265 | ||
| ENC002065 | 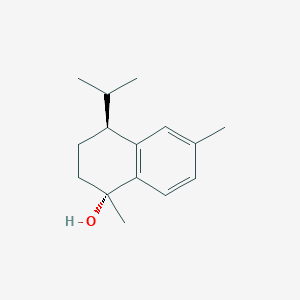 |
0.256 | D0T7ZQ | 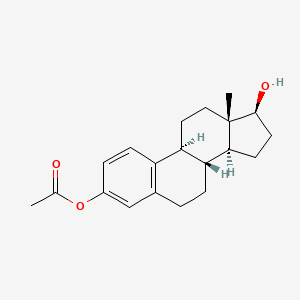 |
0.263 | ||
| ENC003790 |  |
0.256 | D03XES | 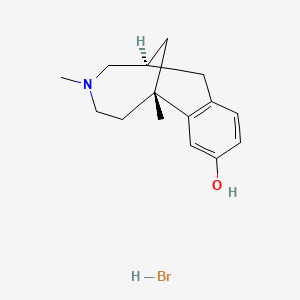 |
0.253 | ||
| ENC002280 | 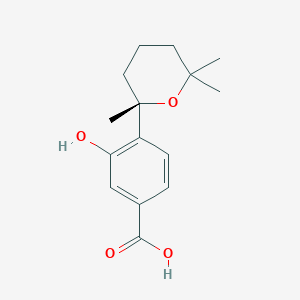 |
0.247 | D0T6WT | 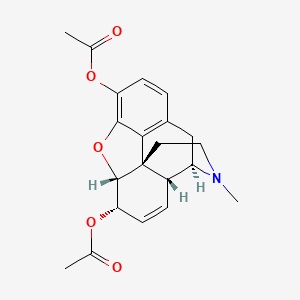 |
0.248 | ||
| ENC002187 | 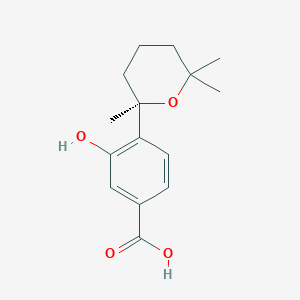 |
0.247 | D08USJ | 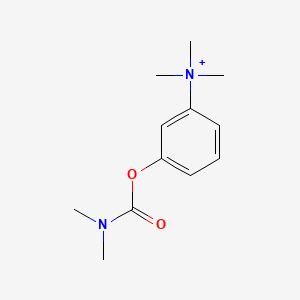 |
0.244 | ||
| ENC004193 | 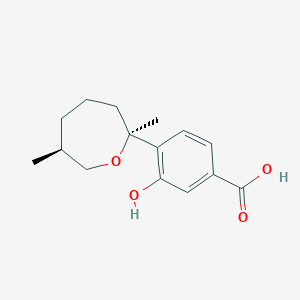 |
0.244 | D02IOH | 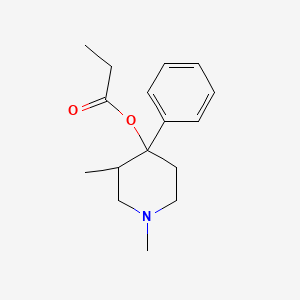 |
0.241 | ||
| ENC004192 | 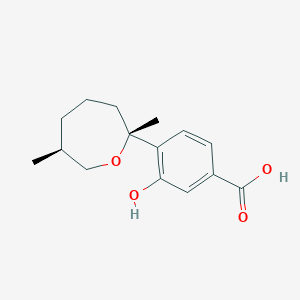 |
0.244 | D0T3HY | 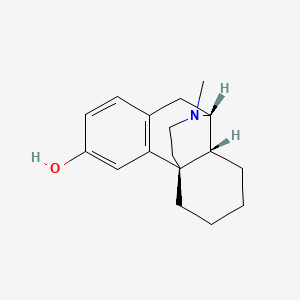 |
0.236 | ||
| ENC004993 | 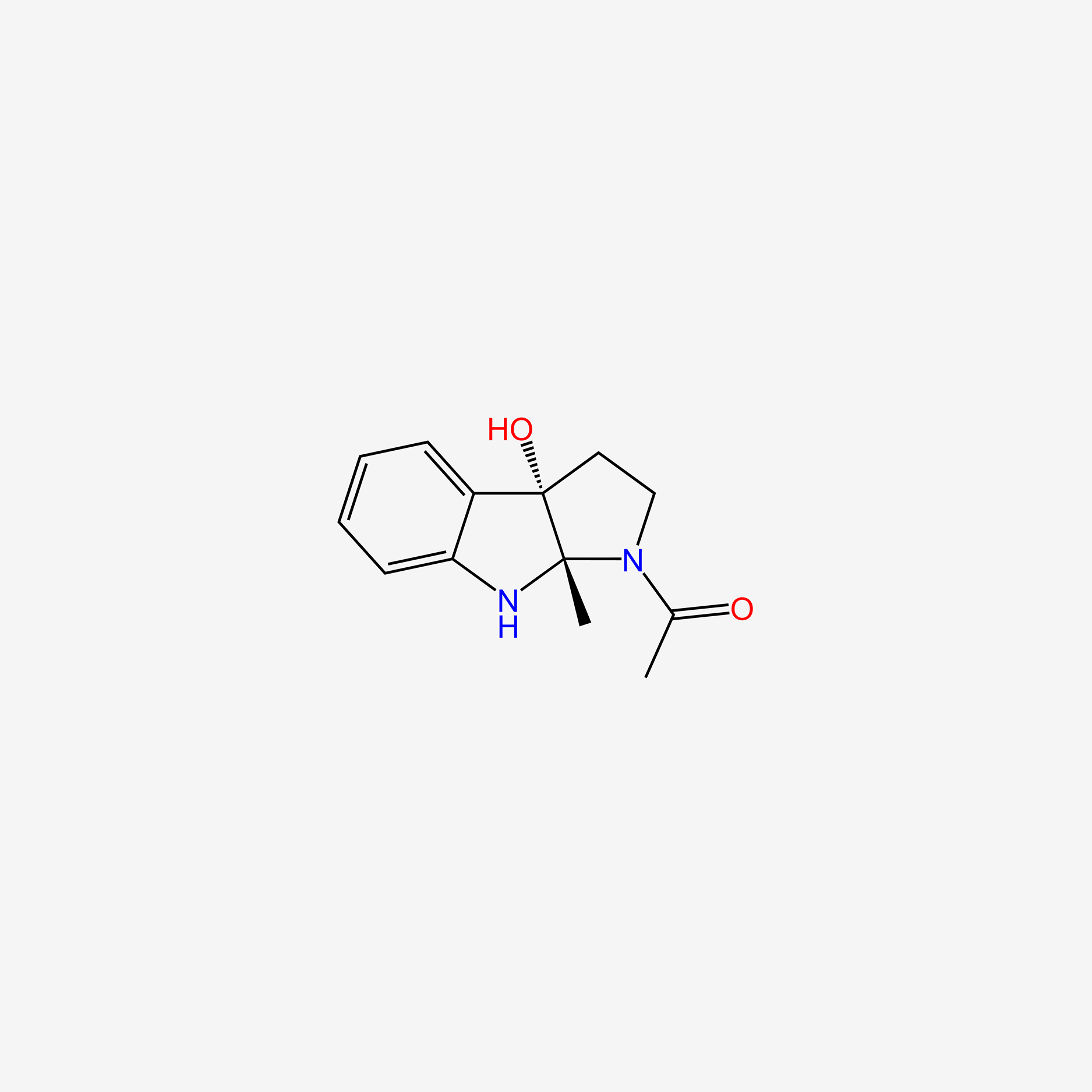 |
0.244 | D02DPU | 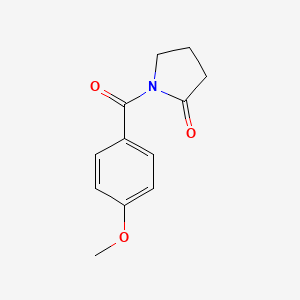 |
0.235 | ||