NPs Basic Information

|
Name |
Tetroquinone
|
| Molecular Formula | C6H4O6 | |
| IUPAC Name* |
2,3,5,6-tetrahydroxycyclohexa-2,5-diene-1,4-dione
|
|
| SMILES |
C1(=C(C(=O)C(=C(C1=O)O)O)O)O
|
|
| InChI |
InChI=1S/C6H4O6/c7-1-2(8)4(10)6(12)5(11)3(1)9/h7-8,11-12H
|
|
| InChIKey |
DGQOCLATAPFASR-UHFFFAOYSA-N
|
|
| Synonyms |
Tetrahydroxyquinone; tetroquinone; 319-89-1; 2,3,5,6-tetrahydroxycyclohexa-2,5-diene-1,4-dione; Tetrahydroxy-1,4-benzoquinone; Kelox; Tetrahydroxy-p-benzoquinone; Terasin; HPEK-1; Tetrahydroxy-p-quinone; Tetrahydroxy-1,4-quinone; Tetrahydroxyparabenzoquinone; Tetroquinona; Tetroquinonum; 2,5-Cyclohexadiene-1,4-dione, 2,3,5,6-tetrahydroxy-; 2,3,5,6-Tetrahydroxy-p-benzoquinone; NSC-112931; U7L4P6H0SU; NSC112931; NCGC00095043-01; p-Benzoquinone, 2,3,5,6-tetrahydroxy-; DSSTox_CID_25897; DSSTox_RID_81209; DSSTox_GSID_45897; Tetrahydroxy-1,4-benzoquinone;Tetrahydroxybenzoquinone; Tetrochinone; Tetrochinone [DCIT]; Tetrahydroxy-1,4-benzoquinone Hydrate; CAS-319-89-1; Tetroquinone [USAN:INN]; UNII-U7L4P6H0SU; Tetroquinonum [INN-Latin]; Tetroquinona [INN-Spanish]; Tetraoxychinon; 2,3,5,6-tetrahydroxy-1,4-benzoquinone; 4-benzoquinone; SR-05000002066; Tetrahydroxy-1; tetrahydroxy quinone; EINECS 206-275-5; NSC 112931; tetrahydroxybenzoquinone; Spectrum_001691; Prestwick0_000835; Prestwick1_000835; Prestwick2_000835; Prestwick3_000835; Spectrum2_000866; Spectrum3_000750; Spectrum4_000847; Spectrum5_001627; TETROQUINONE [MI]; Tetroquinone (USAN/INN); TETROQUINONE [INN]; TETROQUINONE [USAN]; BSPBio_000789; BSPBio_002340; KBioGR_001453; KBioSS_002171; DivK1c_000623; SCHEMBL167587; SPECTRUM1503330; SPBio_000911; SPBio_002710; 2,3,5,6-Tetrahydroxy-2,5-cyclohexadiene-1,4-dione; BPBio1_000869; SCHEMBL4657549; CHEMBL1329029; DTXSID9045897; HMS501P05; KBio1_000623; KBio2_002171; KBio2_004739; KBio2_007307; KBio3_001560; CHEBI:137472; NINDS_000623; HMS1922A16; HMS2093E07; Pharmakon1600-01503330; 2,5,6-Tetrahydroxy-p-benzoquinone; HY-B1106; YEA33416; ZINC1665742; Tox21_111400; CCG-39742; MFCD00001597; NSC758457; p-Benzoquinone,3,5,6-tetrahydroxy-; STL453704; AKOS006228209; Tox21_111400_1; CS-4703; DS-3819; NSC-758457; 2,4-dione, 2,3,5,6-tetrahydroxy-; IDI1_000623; NCGC00095043-02; NCGC00095043-03; NCGC00095043-04; NCGC00095043-07; 2,3,5,6-Tetrahydroxybenzo-1,4-quinone; AC-18203; 2,3,5,6-tetrahydroxy-[1,4]benzoquinone; SBI-0051815.P002; DB-068561; tetrahydroxycyclohexa-2,5-diene-1,4-dione; 2,3,5,6-Tetrahydroxybenzo-1,4-quinone #; FT-0656093; T1090; D06096; F17741; AB00052347_04; A821088; J-018590; Q3546866; SR-05000002066-1; 2,3,5,6-Tetrahydroxy-2,5-cyclohexadien-1,4-dione
|
|
| CAS | 319-89-1 | |
| PubChem CID | 5424 | |
| ChEMBL ID | CHEMBL1329029 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 172.09 | ALogp: | -0.6 |
| HBD: | 4 | HBA: | 6 |
| Rotatable Bonds: | 0 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 115.0 | Aromatic Rings: | 1 |
| Heavy Atoms: | 12 | QED Weighted: | 0.388 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -5.346 | MDCK Permeability: | 0.00018584 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.072 |
| Human Intestinal Absorption (HIA): | 0.015 | 20% Bioavailability (F20%): | 0.004 |
| 30% Bioavailability (F30%): | 0.042 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.402 | Plasma Protein Binding (PPB): | 80.46% |
| Volume Distribution (VD): | 0.43 | Fu: | 12.43% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.02 | CYP1A2-substrate: | 0.056 |
| CYP2C19-inhibitor: | 0.081 | CYP2C19-substrate: | 0.052 |
| CYP2C9-inhibitor: | 0.071 | CYP2C9-substrate: | 0.357 |
| CYP2D6-inhibitor: | 0.008 | CYP2D6-substrate: | 0.117 |
| CYP3A4-inhibitor: | 0.009 | CYP3A4-substrate: | 0.012 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 1.099 | Half-life (T1/2): | 0.234 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.012 | Human Hepatotoxicity (H-HT): | 0.499 |
| Drug-inuced Liver Injury (DILI): | 0.437 | AMES Toxicity: | 0.094 |
| Rat Oral Acute Toxicity: | 0.095 | Maximum Recommended Daily Dose: | 0.001 |
| Skin Sensitization: | 0.745 | Carcinogencity: | 0.003 |
| Eye Corrosion: | 0.25 | Eye Irritation: | 0.979 |
| Respiratory Toxicity: | 0.923 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC003525 | 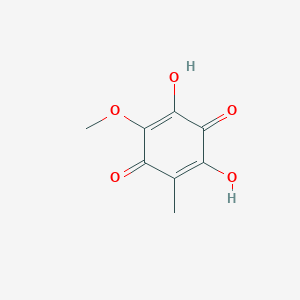 |
0.432 | D07AHW | 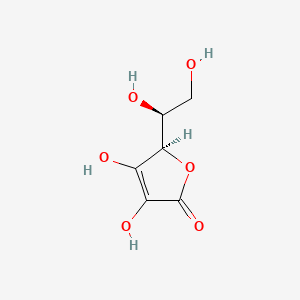 |
0.245 | ||
| ENC000670 | 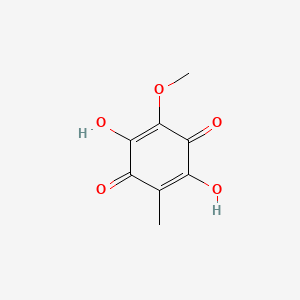 |
0.400 | D04AIT |  |
0.222 | ||
| ENC003505 | 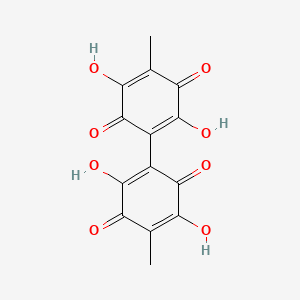 |
0.387 | D0K8KX |  |
0.216 | ||
| ENC001362 | 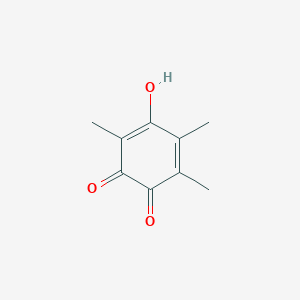 |
0.333 | D07EXH | 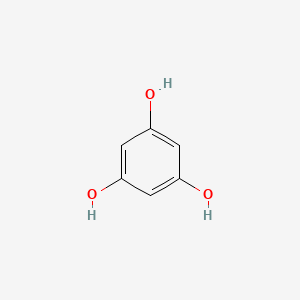 |
0.200 | ||
| ENC000934 | 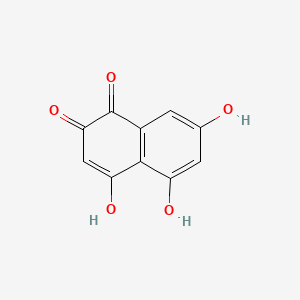 |
0.321 | D0R9WP |  |
0.188 | ||
| ENC000335 | 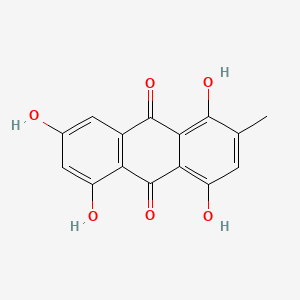 |
0.303 | D0H1AR | 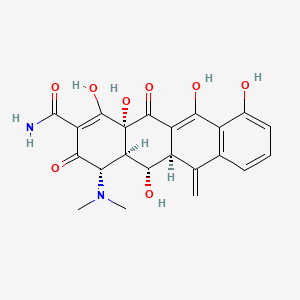 |
0.188 | ||
| ENC000822 | 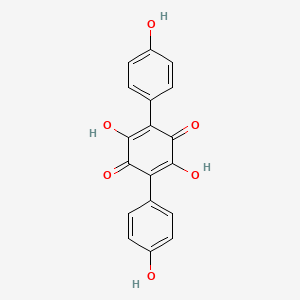 |
0.297 | D08NQZ | 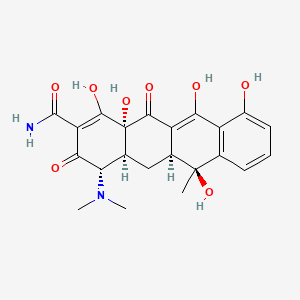 |
0.188 | ||
| ENC001058 | 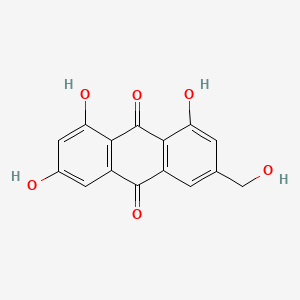 |
0.279 | D0S0LZ |  |
0.188 | ||
| ENC004924 | 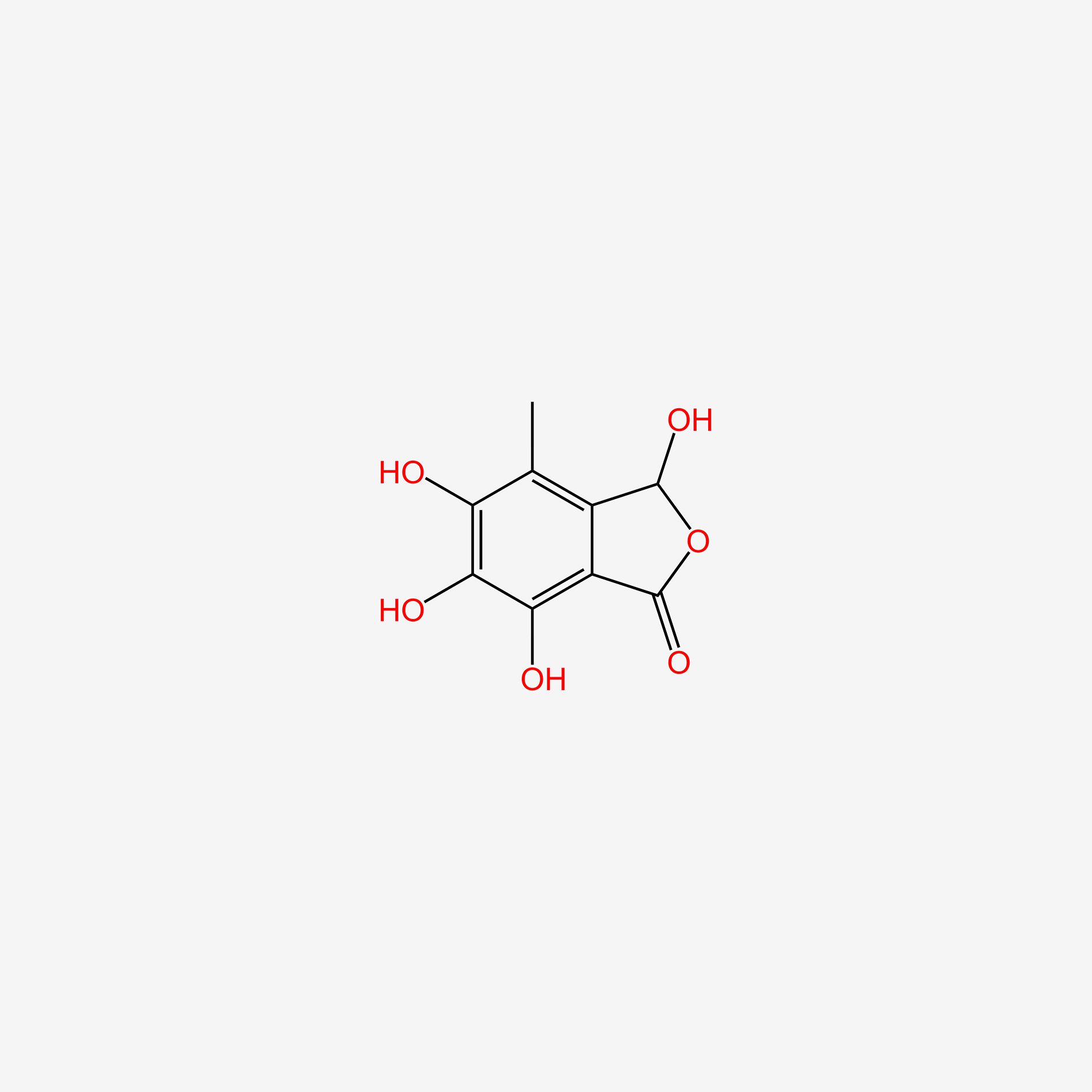 |
0.278 | D0U3YB | 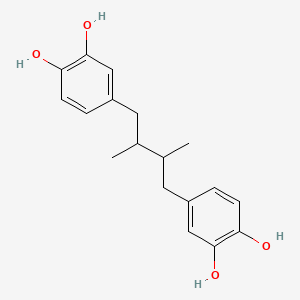 |
0.184 | ||
| ENC002929 | 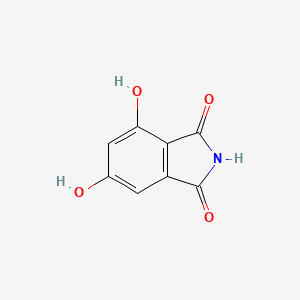 |
0.275 | D00KRE | 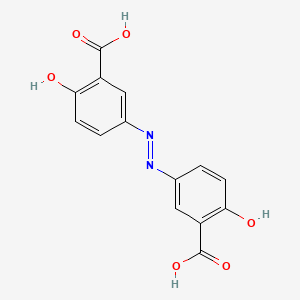 |
0.184 | ||