NPs Basic Information
|
Name |
Isoflurane
|
| Molecular Formula | C3H2ClF5O | |
| IUPAC Name* |
2-chloro-2-(difluoromethoxy)-1,1,1-trifluoroethane
|
|
| SMILES |
C(C(F)(F)F)(OC(F)F)Cl
|
|
| InChI |
InChI=1S/C3H2ClF5O/c4-1(3(7,8)9)10-2(5)6/h1-2H
|
|
| InChIKey |
PIWKPBJCKXDKJR-UHFFFAOYSA-N
|
|
| Synonyms |
isoflurane; 26675-46-7; Forane; 1-Chloro-2,2,2-trifluoroethyl difluoromethyl ether; 2-Chloro-2-(difluoromethoxy)-1,1,1-trifluoroethane; Forene; Aerrane; Isoflurano; Compound 469; IsoFlo; Isofluranum; Ethane, 2-chloro-2-(difluoromethoxy)-1,1,1-trifluoro-; R-E 235dal; Terrell; Ether, 1-chloro-2,2,2-trifluoroethyl difluoromethyl; HSDB 8057; CYS9AKD70P; COMPOUND-469; CHEBI:6015; 2-chloro-2-difluoromethoxy-1,1,1-trifluoroethane; Isofluranum [INN-Latin]; NCGC00181037-01; AErrane (Veterinary); Difluoromethyl 1-chloro-2,2,2-trifluoroethyl ether (Isoflurane); DSSTox_CID_752; DSSTox_RID_75769; DSSTox_GSID_20752; Isoba; Isofor; Isoforine; Isorrane; R-E 235da1; Isoflurano [INN-Spanish]; CAS-26675-46-7; Forane (TN); CCRIS 3043; Isoflurane [Anaesthetics, volatile]; EINECS 247-897-7; UNII-CYS9AKD70P; MFCD00066609; BRN 1852087; (+/-)-Isoflurane; Isoflurane [USAN:USP:INN:BAN:JAN]; TerrellHSDB 8057; Compd 469; Isoflurane, AldrichCPR; ISOFLURANE [MI]; ISOFLURANE [INN]; ISOFLURANE [JAN]; ISOFLURANE [USAN]; ISOFLURANE [VANDF]; ISOFLURANE [MART.]; SCHEMBL1532; CHEMBL1256; ISOFLURANE [USP-RS]; ISOFLURANE [WHO-DD]; difluoromethyl 1-chloro-2,2,2-trifluoroethyl ether; GTPL2505; HSDB8057; Isoflurane (JP17/USP/INN); ISOFLURANE [GREEN BOOK]; DTXSID3020752; ISOFLURANE [ORANGE BOOK]; HSDB-8057; ISOFLURANE [EP MONOGRAPH]; BDBM217353; ISOFLURANE [USP MONOGRAPH]; AMY33546; Tox21_112685; Tox21_200831; BBL100111; s6917; STL454337; AKOS006228574; DB00753; KS-5166; PB47772; NCGC00181037-02; NCGC00181037-03; NCGC00258385-01; AC-154802; DB-046999; CS-0017450; FT-0627416; C07518; D00545; EN300-123043; P15338; A818554; Q413918; SR-01000944965; SR-01000944965-1; W-107162; 1-Chloro-1-(difluoromethoxy)-2,2,2-trifluoroethane; 2-chloro-2-(difluoromethoxy)-1,1,1-trifluoro-ethane; 1-CHLORO-2,2,2-TRIFLUROETHYL DIFLUROMETHYL ETHER; Ethane, 1-chloro-1-(difluoromethoxy)-2,2,2-trifluoro-; Z1201618663; 2-[bis(fluoranyl)methoxy]-2-chloranyl-1,1,1-tris(fluoranyl)ethane
|
|
| CAS | 26675-46-7 | |
| PubChem CID | 3763 | |
| ChEMBL ID | CHEMBL1256 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 184.49 | ALogp: | 2.1 |
| HBD: | 0 | HBA: | 6 |
| Rotatable Bonds: | 2 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 9.2 | Aromatic Rings: | 0 |
| Heavy Atoms: | 10 | QED Weighted: | 0.473 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.379 | MDCK Permeability: | 0.00003600 |
| Pgp-inhibitor: | 0.001 | Pgp-substrate: | 0.001 |
| Human Intestinal Absorption (HIA): | 0.003 | 20% Bioavailability (F20%): | 0.011 |
| 30% Bioavailability (F30%): | 0.001 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.985 | Plasma Protein Binding (PPB): | 90.02% |
| Volume Distribution (VD): | 1.111 | Fu: | 16.14% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.855 | CYP1A2-substrate: | 0.943 |
| CYP2C19-inhibitor: | 0.068 | CYP2C19-substrate: | 0.85 |
| CYP2C9-inhibitor: | 0.052 | CYP2C9-substrate: | 0.025 |
| CYP2D6-inhibitor: | 0.007 | CYP2D6-substrate: | 0.123 |
| CYP3A4-inhibitor: | 0.01 | CYP3A4-substrate: | 0.234 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 8.142 | Half-life (T1/2): | 0.281 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.024 | Human Hepatotoxicity (H-HT): | 0.973 |
| Drug-inuced Liver Injury (DILI): | 0.418 | AMES Toxicity: | 0.009 |
| Rat Oral Acute Toxicity: | 0.029 | Maximum Recommended Daily Dose: | 0.228 |
| Skin Sensitization: | 0.059 | Carcinogencity: | 0.68 |
| Eye Corrosion: | 0.003 | Eye Irritation: | 0.014 |
| Respiratory Toxicity: | 0.037 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
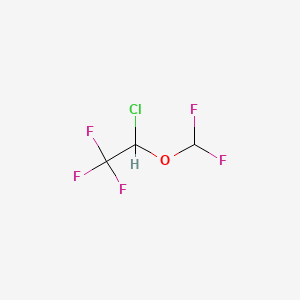
| ENC002390 | 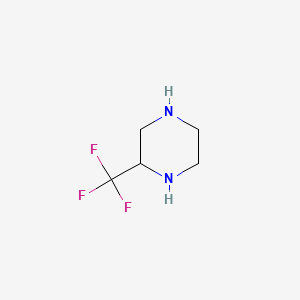 |
0.159 | D0DP6L | 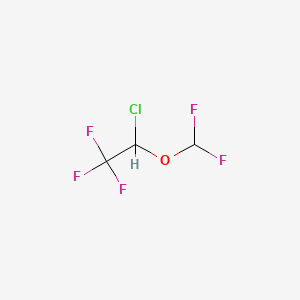 |
1.000 | ||
| ENC000150 | 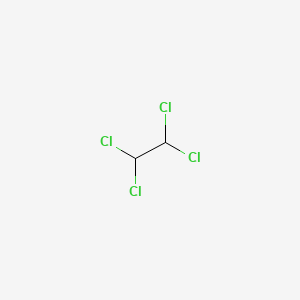 |
0.121 | D0H4GN | 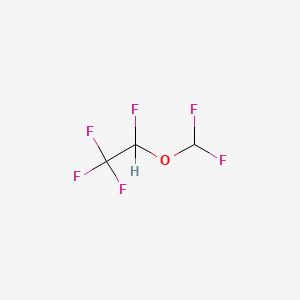 |
0.600 | ||
| ENC001194 | 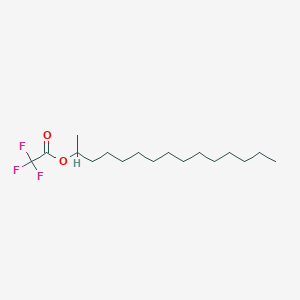 |
0.120 | D0AO9S | 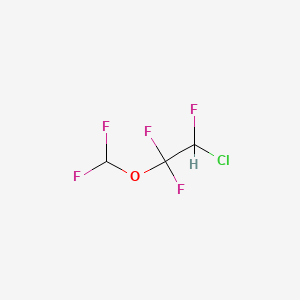 |
0.500 | ||
| ENC001157 | 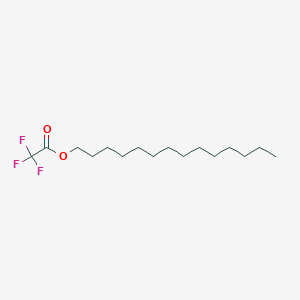 |
0.108 | D0D8VE | 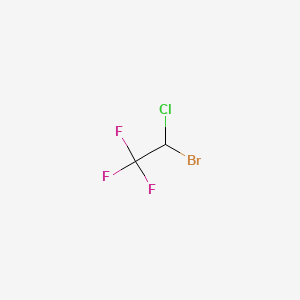 |
0.393 | ||
| ENC003065 | 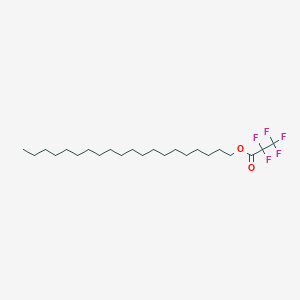 |
0.103 | D0W6ZF | 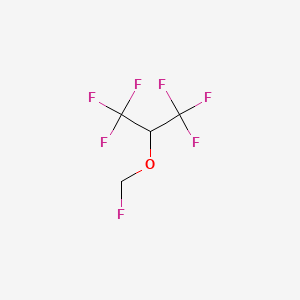 |
0.293 | ||
| ENC003066 | 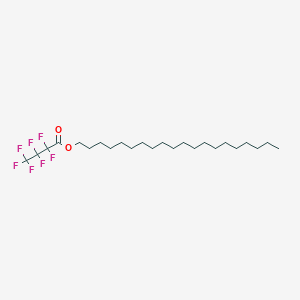 |
0.096 | D07SOO | 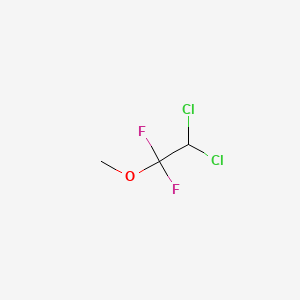 |
0.265 | ||
| ENC001259 | 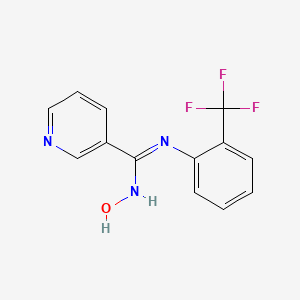 |
0.096 | D0G5BK | 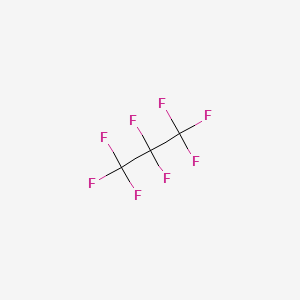 |
0.225 | ||
| ENC003041 | 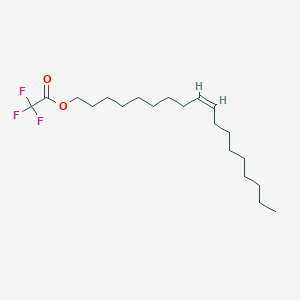 |
0.093 | D0H0KB | 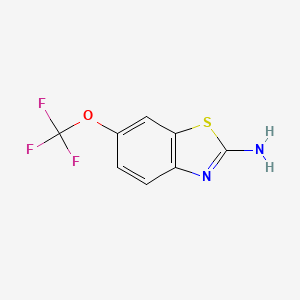 |
0.140 | ||
| ENC001163 | 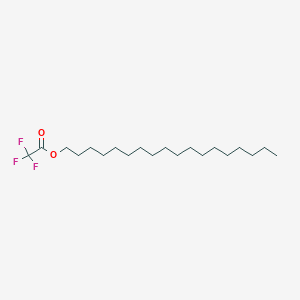 |
0.093 | D0X7JR | 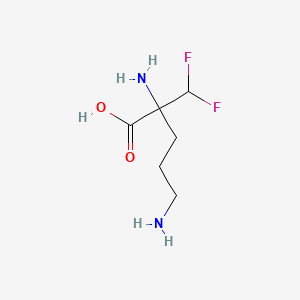 |
0.125 | ||
| ENC002300 | 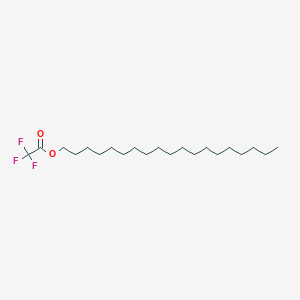 |
0.090 | D0Y0SW | 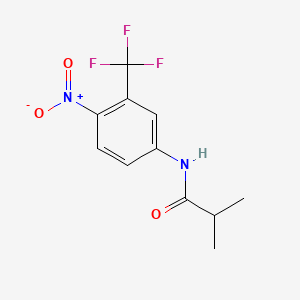 |
0.123 | ||